Zusammenfassung
Die Paläoökologie des Riesenhirsches (Megaloceros giganteus) einschließlich seiner Ernährungspräferenzen ist wenig bekannt, vor allem, weil Rekonstruktionen auf der Grundlage morphologischer Merkmale zu widersprüchlichen Ergebnissen geführt haben. In dieser Studie schlagen wir vor, anhand der Analysen von Mikro-Abnutzungsspuren (engl. microwear) und Meso-Abnutzungsspuren (engl. mesowear) an Zähnen die Ernährung der Riesenhirsche aus fünf archäologischen Fundstätten in Süddeutschland und im Harz zu rekonstruieren. Berücksichtigt werden außerdem die Ergebnisse einer Überprüfung von Daten zu Zahnabnutzungsspuren, die während des letzten Jahrzehnts für zehn Orte in Europa veröffentlicht wurden. Ziel ist es, einen großräumigen Einblick in die Ernährung des Riesenhirsches zu geben und die räumliche und zeitliche Vielfalt seiner Ernährungsgewohnheiten zu analysieren. Unseren Ergebnissen zufolge reichten die Ernährungsweisen des Riesenhirsches von Blattfressen (engl. leaf browsing) [Anm. d. Red.: browsers sind Tiere, die spezialisiert sind auf das Fressen von Blättern, Früchten hochwachsender Gehölze sowie weicher Triebe und Sträucher. Im Gegensatz dazu sind grazers Herbivoren, die sich von Pflanzen wie Gras und anderer tiefliegender Vegetation ernähren] bis hin zu grasdominierter Mischernährung, abhängig von der regional und saisonal verfügbaren Vegetation. Die Kombination der beiden Anzeiger Meso-Abnutzung (mesowear) und Mikro-Abnutzung (microwear) ermöglichte es uns, die Flexibilität bei der Ernährung des Riesenhirsches zu charakterisieren. Schließlich diskutieren wir die Ursachen seiner Ausrottung und kommen zu dem Schluss, dass sein Aussterben wahrscheinlich nicht durch eine zu enge Ernährungsnische herbeigeführt wurde.
Introduction
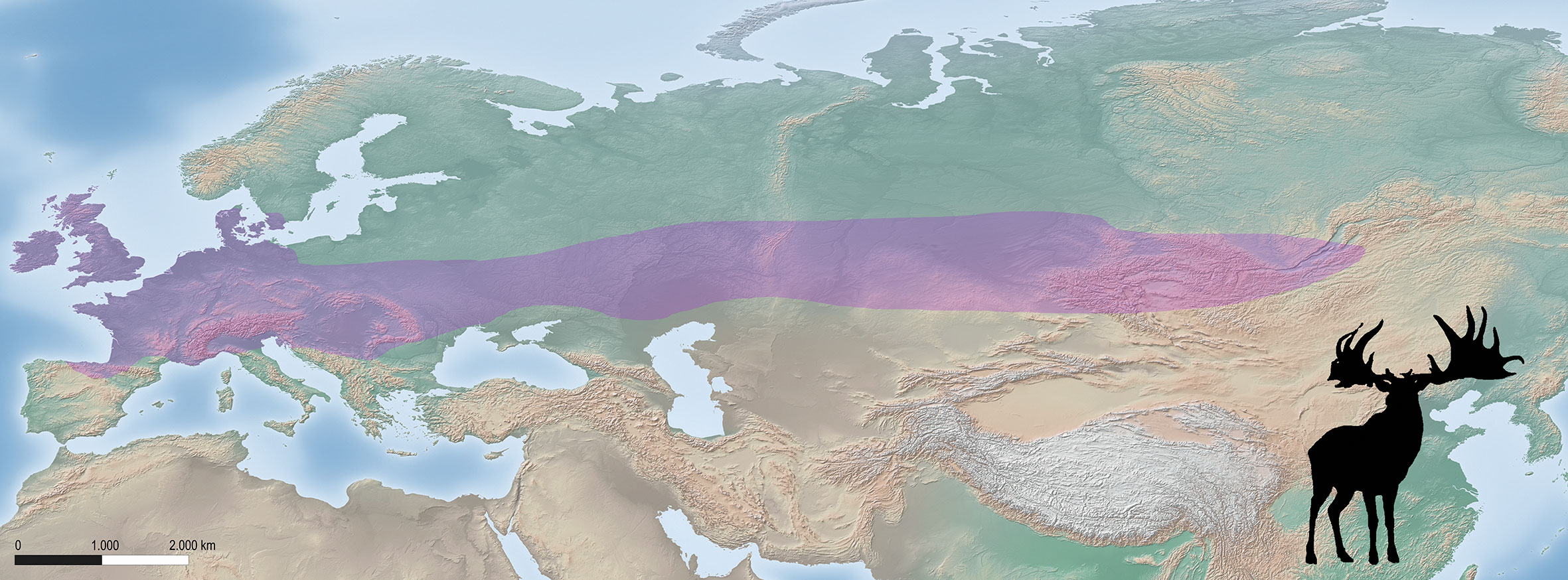
During the Late Pleistocene, the distribution range of the giant deer, Megaloceros giganteus, occupied a significant area across Europe and Western Asia (Lister 1981, 1994) (Fig. 1). The species was adapted to a wide variety of climatic conditions, from interglacial periods to boreal or subarctic habitats during glacial stages (Lister 1994). The giant deer, as with the other megacerines known in the Pleistocene, lacks modern analogues in extant ecosystems. Consequently, it is difficult to establish the ecological characteristics of this species. Most of the studies that proposed paleoecological reconstructions were based on the examination of skeletal morphological features related to diet, including the morphology of the skull and lower jaw (e.g., longer diastema), tooth crown height (e.g., mesodont teeth), shape and position of the incisors, or the degree of molarization of the premolars (Lister 1994; Vislobokova 2012). The application of these proxies to assemblages from diverse localities resulted in discrepant interpretations, and different hypotheses were raised about the paleodiet and the habitats of the giant deer. Following Kahlke (1999), it was primarily a grazer and mostly occupied open habitats, such as wooded steppes or steppe-like biotopes. Conversely, Aaris-Sørensen and Liljegren (2004) classified the giant deer principally as a browser that complemented its diet with grass and compared its feeding ecology to that of the elk (Alces alces). However, mesowear and microwear values classify the elk as a pure leaf browser, both in Europe (Rivals et al. 2010) and North America (Fortelius and Solounias 2000; Solounias and Semprebon 2002). A compromise, proposed by Stuart et al. (2004), suggests that giant deer were mixed feeders, depending both on grass and shrubs (i.e., a generalist species) in open woodlands. The fossil record indicates that M. giganteus is not associated with the steppe, unlike woolly mammoths, reindeer or other cold-adapted species. During the last glaciation, the giant deer distribution corresponded to an area of boreal parkland (Pushkina and Raia 2008; Lister and Stuart 2019) with vegetation composed of dispersed pine or spruce, shrubs and other plants, such as grasses, sedges, Artemisia, Ephedra and Chenopodiaceae (Allen et al. 2010). Dental morphology (i.e., mesodont teeth) suggests that the giant deer was adapted to mixed feeding and that the vegetation available allowed this species to feed both on grass and browse (Lister and Stuart 2019).
Throughout the Late Cenozoic, the evolutionary trend of the megacerines corresponds to a transition from forested habitats to more open ones, which is related to a shift in the diet of browsing species towards more grazing (i.e., including more grass in their diet) (Vislobokova 2012, 714). Even if giant deer were classified as mixed feeders based on morphological features (Stuart et al. 2004; Vislobokova 2012, 717) and adaptations to grazing may have increased over time (Lister 1994), recent studies show a different picture of the diet of megacerines (Rivals and Lister 2016). It is critical to consider that morphology may represent a superimposition of actual ecomorphological and morpho-functional adaptations and features inherited from ancestral forms, as, for instance, in the case of the cervid Haploidoceros mediterraneus (Croitor et al. 2020). In the case of opportunistic species (e.g., most of the cervids), ecomorphology provides clues about the general adaptations within a lineage, but not the exact ecology of individuals or populations. Studies on single assemblages usually show very diverse results because the data reflect specific dietary traits depending on the local environmental context. For example, stable carbon isotopes in Irish giant deer indicate a diet based on herbaceous plants complemented with woody browse (Chritz et al. 2009). The plant remains preserved in the molar infundibulum of a single giant deer molar from the North Sea were composed of Artemisia (sage) and other Asteraceae, indicating browsing habits in steppe habitats (van Geel et al. 2018).
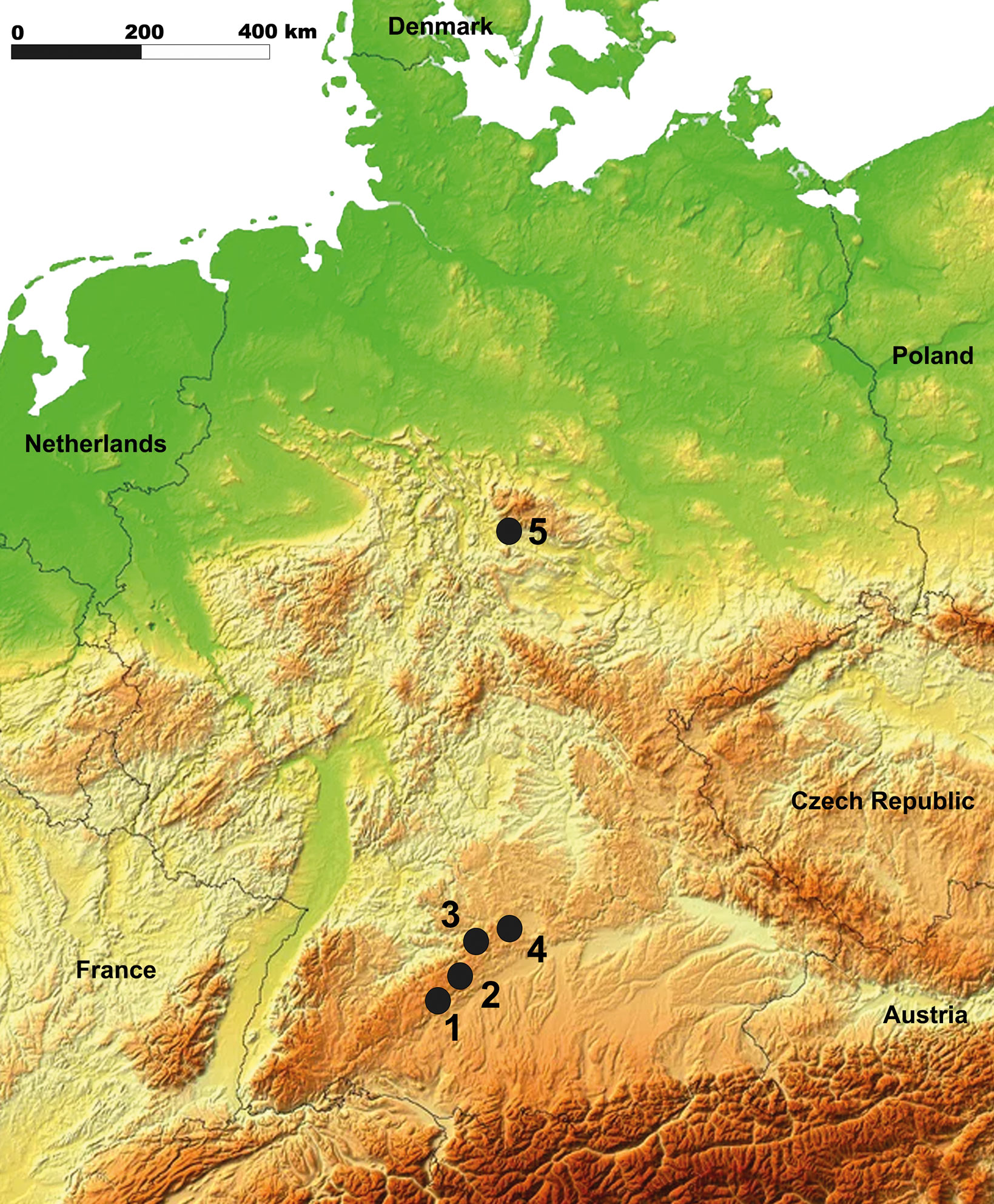
The diet of the giant deer is an important aspect of the debate concerning its extinction due to niche partitioning with other cervids (Immel et al. 2015). However, reconstructions based on morphological data have produced contradictory results, and the palaeoecology of the giant deer, including its dietary preferences, is still poorly known. Most of the previous research is based on local or regional studies that may not reflect the spatial and temporal diversity of the dietary habits of the giant deer. In this study, we propose to reconstruct the giant deer diet from five sites located in Southern Germany and the Harz Mountains (Fig. 2) using microwear and mesowear analyses and to include the results in a review of the tooth wear data published during the past decade for ten localities in Europe. We also include the analysis of the carbon, nitrogen and sulfur stable isotopes sampled from the giant deer from Wolftalhöhle. The objective is to provide a large-scale vision of the diet of the giant deer across Europe during the Late Pleistocene.
Materials and Methods
Materials
All the dental specimens available from Groβe Ofnet (n = 14), Vogelherd (n = 5), Einhornhöhle layers 4.5 and 5 (n = 3), Wolftalhöhle (n = 1) and Geißenklösterle (n = 1) were selected and sampled for mesowear and microwear analyses (Fig. 3).
Einhornhöhle (Unicorn Cave) is located near the town of Herzberg in the southern Harz Mountains at about 380 m asl and developed in the dolomitic rock of the Permian Zechstein formation (Kaufmann et al. 2020). After the initial discovery of Middle Paleolithic artifacts in 1985 (the Jacob-Friesen Gallery), succeeding excavations exposed six Middle Paleolithic layers inside the gallery and two further ones at a former cave entrance connected to the gallery (Kotula et al. 2019). The giant deer teeth were discovered in layers 4.5 and 5 of this former cave entrance. Animal remains of these layers mostly come from Ursus spelaeus, Bison sp., and Megaloceros giganteus. An outstanding item from layer 4.5 is the engraved giant deer phalanx dating to >55,000–47,492 cal BP (ca. 47.8 ka BP, KIA-55192), which can be viewed in the context of Neanderthal symbolic behavior (Leder et al. 2021). The giant deer teeth analyzed herein might belong to the same individual as the engraved phalanx and the other giant deer remains (NISP = 19, MNI = 1), and therefore should be of the same age. In layer 5, the giant deer remains (NISP = 2, MNI = 1) correspond to a single individual.
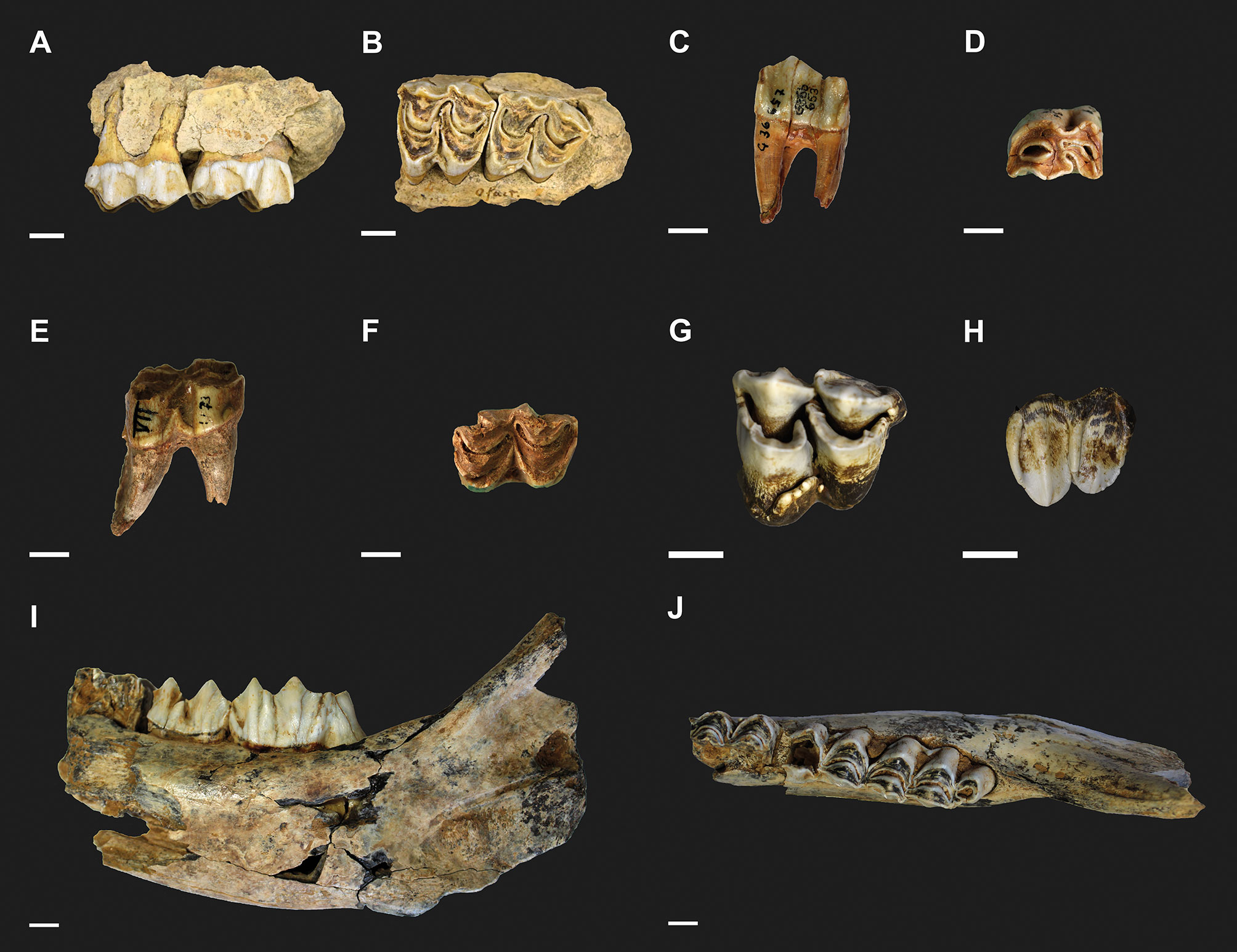
Wolftalhöhle (Wolftal Cave) is located in a small side valley just north of the Lone near Bernstadt. In the spring of 2015, a team from the University of Tübingen led by N. J. Conard excavated a test trench of 3 m² in this small cave (Conard et al. 2016). The team identified four strata, all lacking Paleolithic artifacts over a maximum depth of 1.5 m. At the base of the deepest layer, just above the bedrock, lay a well-preserved mandible from an adult giant deer. Radiocarbon dating produced an age in excess of ca. 48,700 BP (>49,588-48,007 cal BP, OxA-33575), which corresponds to a minimum age for the giant deer and the base of the sequence. The upper layers of this small cave contained ceramics, including material from the Late Bronze Age.
Vogelherd, situated near the city of Niederstotzingen, is probably the richest of the many Paleolithic cave sites in the Swabian Jura. This moderately sized cave with an area of 170 m² is located at an elevation of 477 m asl and stands 18 m above the floor of the Lone Valley (Conard et al. 2015). G. Riek (1934) excavated the site completely in the summer of 1931 and recovered a dozen Aurignacian figurines and many other remarkable finds from the Pleistocene (Riek 1934). N. J. Conard led excavations in the backdirt of Vogelherd from 2005–2012 that greatly expanded the assemblages from all periods recovered from the site. As is typical for the Swabian caves, excavators recovered rich faunal assemblages from the Middle and Upper Paleolithic that provide abundant information on past environments and human subsistence (Niven 2006). The oldest stratum dates to the Eemian Interglacial, roughly 125 ka BP, and the site preserves material from multiple Middle Paleolithic horizons and all the major phases of the Upper Paleolithic. The extremely rich Aurignacian deposits yielded by far the largest assemblages of figurative art and personal ornaments from the Swabian Jura, as well as multiple fragments of musical instruments. In 2017, Vogelherd was awarded the status of a UNESCO World Cultural Heritage Site together with five other caves from the Ach and Lone Valleys.
Große Ofnet is located on a limestone hill in the Swabian Jura near the town of Nördlingen in Bavaria. The cave contains a 2-m-thick stratigraphic sequence that was excavated by O. Fraas in 1875 and by R. R. Schmidt in 1907–1908 (Uthmeier 2004). The materials available at the Staatliches Museum für Naturkunde Stuttgart (SMNS) come from the Fraas excavation and lack stratigraphic information. The fauna includes Coelodonta antiquitatis, Equus ferus, Megaloceros giganteus, Bison priscus and Ursus spelaeus. Schmidt (1912) identified four archaeological layers belonging to the Upper Palaeolithic: early Aurignacian (layer III), late Aurignacian (layer IV), Solutrean (layer V) and Magdalenian (layer VI). The presence of giant deer was only reported in the Aurignacian layers, without attributing the remains to either layer III or layer IV (Koken 1912). The sample will be considered a single assemblage belonging to the Aurignacian.
Geißenklösterle is a partially collapsed cave that stands 60 meters above the base of the Ach Valley near the city of Blaubeuren at an elevation of 585 m asl (Hahn 1988; Conard et al. 2015, 2019). While the site is best known for its Aurignacian assemblages, including carved ivory figurines and musical instruments, the site preserves a stratigraphic sequence from the Middle Paleolithic dated with ESR to between ca. 95 and 45 ka BP (Richard et al. 2019). Multiple techniques, most importantly radiocarbon, date the rich Aurignacian and Gravettian deposits between ca. 43 and 30 ka BP (Higham et al. 2012), and a sparse archaeological signature from the Magdalenian dates after the Last Glacial Maximum (LGM) to ca 15 ka BP. Mesolithic finds have also been recovered at Geißenklösterle. The site was initially excavated by E. Wagner in 1973, and in the years 1974-1983 and 1986-1991, J. Hahn (1988) excavated the site. In 2001 and 2002, N. J. Conard headed a dig focusing on the deeper horizons and reaching bedrock (Conard et al. 2019). Among other topics, the site is well-known for a group of lithic refits from the Gravettian that link Geißenklösterle with three other sites in the Ach Valley (Moreau 2009). The early dates for the Aurignacian and the site’s early symbolic artifacts have placed it at the centre of the debate over the expansion of modern humans into Europe and the extinction of Neanderthals.
Tooth mesowear analysis
Mesowear analysis is a method of categorizing the gross dental wear of ungulate molars by evaluating the relief and sharpness of cusp apices in ways that are correlated with the relative amounts of attritive and abrasive dental wear (Fortelius and Solounias 2000). Mesowear is scored macroscopically from the buccal side of molars. A diet with low levels of abrasion (high attrition) maintains sharpened apices on the buccal cusps as the tooth wears. In contrast, high levels of abrasion, associated with a diet of siliceous grass, result in more rounded and blunted buccal cusp apices. Unworn (and marginally worn) teeth, extremely worn teeth and those with broken or damaged cusp apices are omitted from mesowear analysis. Cusp sharpness is sensitive to ontogenetic age among young individuals (which have not yet developed substantial wear facets) and dentally senescent individuals. However, for intermediate age groups, mesowear is found to be less sensitive to age and more strongly related to diet (Rivals et al. 2007); therefore, it is suitable for dietary reconstruction. The method is based on seven cusp categories (numbered from 0 to 6), ranging in shape from high and sharp (stage 0) to completely blunt with no relief (stage 6) (Mihlbachler et al. 2011). The average value of the mesowear data from a single sample of fossil dentitions corresponds to the ‘mesowear score’ or MWS (Mihlbachler et al. 2011). To reduce inter-observer error, dental mesowear analysis was conducted by a single experienced researcher (FR).
Tooth microwear analysis
Microwear features of dental enamel were examined using a stereomicroscope on high-resolution epoxy casts of teeth following the cleaning, moulding, casting and examination protocol developed by Solounias and Semprebon (2002) and Semprebon et al. (2004). The occlusal surface of each specimen was cleaned using acetone followed by 96% ethanol. The surface was moulded using high-resolution silicone (vinylpolysiloxane), and casts were created using clear epoxy resin. All casts were carefully screened under the stereomicroscope. Those with badly preserved enamel or taphonomic defects (features with unusual morphology and size or fresh features made during the collecting process or during storage) were removed from the analysis, following King et al. (1999). Casts were observed under incident light with a Zeiss Stemi 2000C stereomicroscope at 35× magnification, using the refractive properties of the transparent cast to reveal microfeatures on the enamel. Microwear scars (i.e., elongated scratches and rounded pits) were quantified on the paracone of the upper teeth in a square area of 0.16 mm² using an ocular reticule. We used the classification of features defined by Solounias and Semprebon (2002) and Semprebon et al. (2004), which basically distinguishes pits and scratches. Pits are microwear scars that are circular or sub-circular in outline and thus have approximately similar widths and lengths, while scratches are elongated microfeatures that are not merely longer than they are wide, but have straight, parallel sides. Scratches and pits were counted in two areas either on the paracone of the upper molars or the protoconid of the lower molars. The analysis was conducted by a single experienced researcher (FR).
Comparison with data on giant deer from other localities
The meso- and microwear results were compared with a database constructed from extant ungulate taxa (Fortelius and Solounias 2000; Solounias and Semprebon 2002; Rivals et al. 2010) to establish the following dietary categories: leaf browsers, grazers and mixed feeders. To evaluate the dietary flexibility of the giant deer, the results were compared with data from other localities across Europe: Payre (MIS 5, France: Rivals et al. 2009), La Rexidora (MIS 3, Spain: Rivals and Álvarez-Lao 2018), Steinheim (MIS 11, Germany: Rivals and Ziegler 2018), Siuren I (sample from early Upper Paleolithic (MIS 3), Ukraine: Ramírez-Pedraza et al. 2020), Grays Thurrock (MIS 9), Ilford (MIS 7), Kent’s Cavern (MIS 3, UK: Rivals and Lister 2016), various localities in the Netherlands and at Bruine Bank in the North Sea (MIS 3: Rivals et al. 2010). Unpublished data from the Irish late-glacial population dating to the Allerød interstadial, ca. 11 ka BP, were also included.
Stable carbon nitrogen and sulfur isotopes
The giant deer mandible from Wolftalhöhle was analysed for stable carbon, nitrogen and sulfur isotopes, which depend on the diet and habitat of the studied specimen. In short, the carbon13 abundances (ẟ13C) reflect the source of diet (i.e., the plants consumed by herbivores) over years of the life of the individual when bone collagen is considered. In the context of the Late Pleistocene in Europe, available forage is composed of C3 plants, the 13C abundances of which depend on the conditions of photosynthesis. The environmental conditions of open areas (high light intensity, low humidity, high ventilation conditions) compared with those of forested areas (low light intensity, high humidity, low ventilation conditions) lead to higher and lower ẟ13C values, respectively (e.g., Bocherens 2003). The nitrogen-15 abundances (ẟ15N) in herbivores’ collagen depend on dietary, physiological and climatic conditions. In an adult specimen, climate and diet are the main driving parameters – values of ẟ15N are expected to be lower in cold and humid conditions and/or with consumption of leaves (browsing) in comparison with warm and dry conditions and/or consumption of graminoids (grazing) (e.g., Bocherens 2003). Finally, the sulfur-34 abundances in plants are primarily controlled by the geochemical composition of the soil, itself under the influence of the underlying bedrock, as well as atmospheric depositions (e.g., sea spray effect). The bioavailable sulfur is then transferred to the bone collagen of herbivores through the consumption of plants without a noticeable shift in the ẟ34S values (e.g., Richards et al. 2001).
Collagen was extracted following a protocol based on the acid-alkali-acid procedure (Bocherens et al. 1991). Elemental analysis (Ccoll, Ncoll, Scoll) and isotopic analysis (ẟ13C coll, ẟ15Ncoll, ẟ34Scoll) were conducted at the Department of Geosciences of Tübingen University using a NC2500 CHN-elemental analyser coupled to a Thermo Quest Delta+ XL mass spectrometer. The international standards are a marine carbonate (V-PDB) for ẟ13C, atmospheric nitrogen (AIR) for ẟ15N and Canyon Diablo Troilite (V-CDT) for ẟ34S. Measurements were normalized to ẟ13C values of USGS24 (–16.0‰) and to ẟ15N values of IAEA 305A (+39.8‰). Analytical error, based on the within-run replicate measurement of laboratory standards (albumen, modern collagen, USGS 24, IAEA 305A), was ±0.1‰ for ẟ13C values and ±0.2‰ for ẟ15N values. Samples were calibrated to ẟ34S values of NBS 123 (+17.1‰), NBS 127 (+20.3‰), IAEA-S-1 (+ 0.3‰) and IAEA-S-3 (–32.3‰). The reproducibility was ±0.4‰ for ẟ34S measurements, and the error in the measurement of the amount of S was 5%. The reliability of the ẟ13Ccoll, ẟ15Ncoll and ẟ34Scoll values was established by measuring the collagen chemical composition, with the C:Ncoll atomic ratio ranging from 2.9 to 3.6 (DeNiro 1985) and the C:Scoll and N:Scoll ratios ranging from 300 to 900 and 100 to 300, respectively (Nehlich and Richards 2009).
Results
Tooth mesowear analysis
After discarding the teeth with broken or damaged cusps, the mesowear analysis was performed on the following samples: Groβe Ofnet (n = 12), Einhornhöhle (n = 3), Vogelherd AH VII (n = 4) and AH III (n = 1) and Wolftalhöhle (n = 1). The single tooth from Geißenklösterle, a lower p4, was not suitable for mesowear analysis.
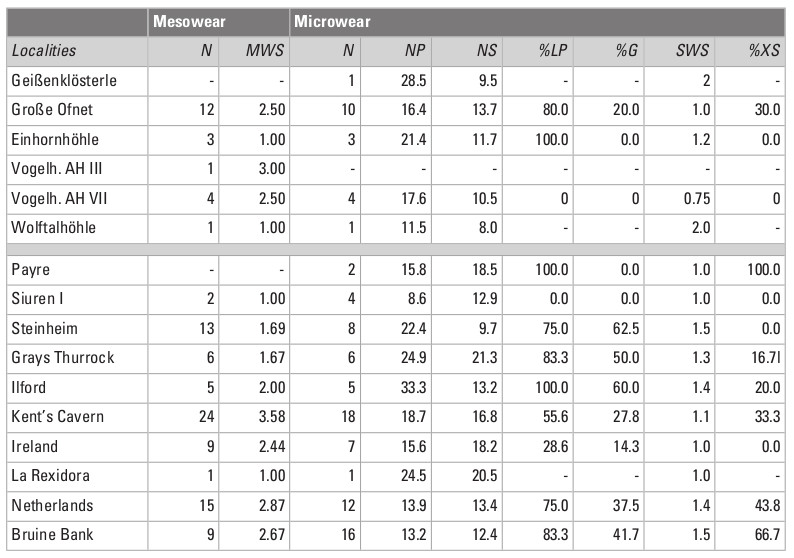
Mesowear scores (MWS) from Groβe Ofnet range between 1 and 5, with a mean of 2.5 (Table 1). The giant deer samples from Vogelherd had mesowear values of 2 and 3, with a mean of 2.6, similar to that of Groβe Ofnet. In comparison to the extant ungulates, the mesowear values of the giant deer from these two sites overlap with those of the grazers, mixed feeders and browsers (Fig. 4). The other two samples, from Einhornhöhle and Wolftalhöhle, are smaller, and the results should be taken with caution. All the mesowear values recorded for Einhornhöhle and Wolftalhöhle were 1. The values overlap with the extant leaf browsers and with the lower values of the mixed feeders (i.e., the browse-dominated mixed feeders). The giant deer from Groβe Ofnet and Vogelherd had a more abrasive diet than those from Einhornhöhle and Wolftalhöhle.
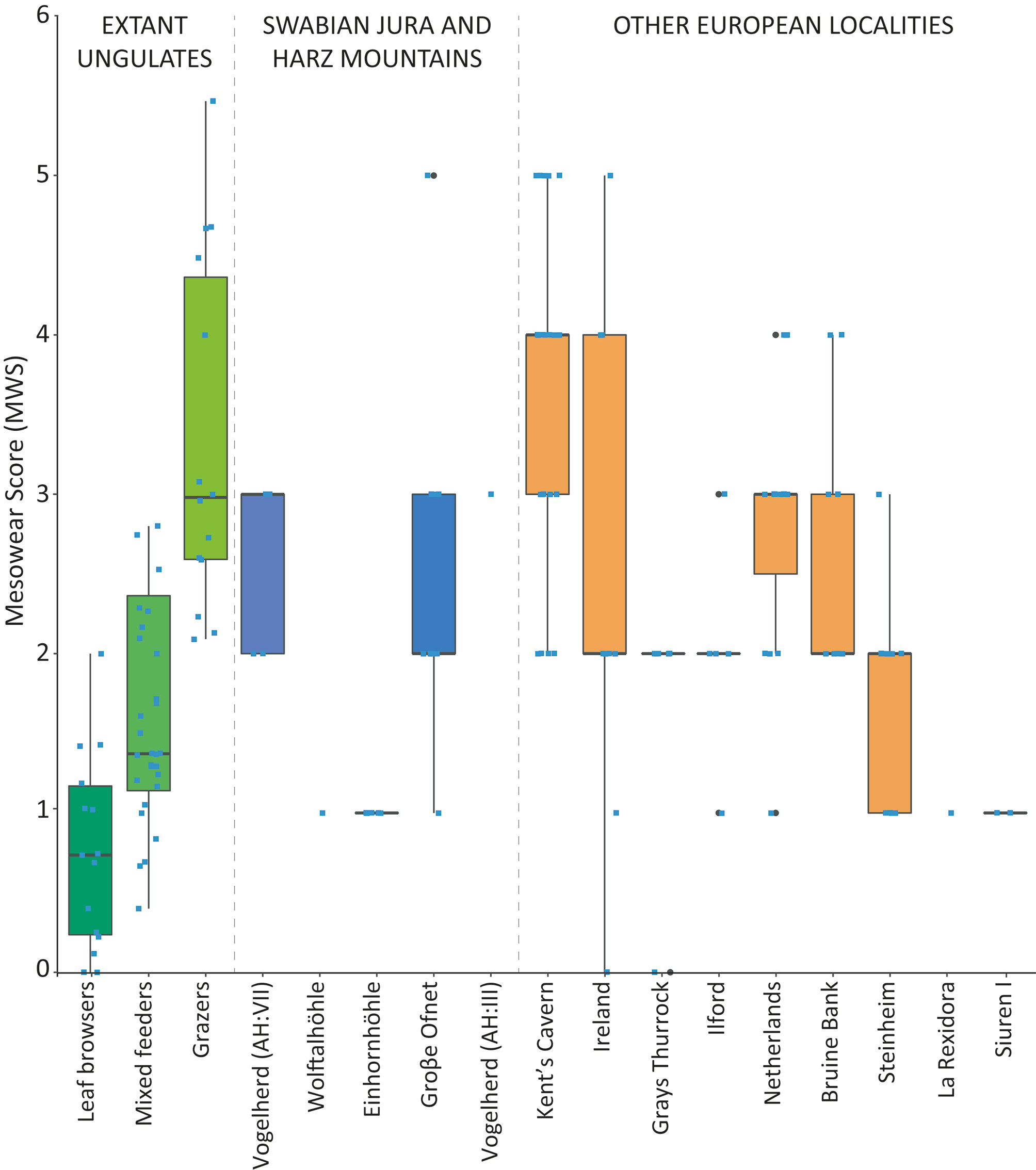
The fossil samples from other localities in Europe show a large diversity of mesowear values, overlapping with the three dietary categories of the extant ungulates (Fig. 4). The samples from Kent’s Cavern, Ireland, the Netherlands and Bruine Bank show relatively high values, and those from La Rexidora and Siuren I show the lowest values, similar to those from Einhornhöhle and Wolftalhöhle.
Tooth microwear analysis
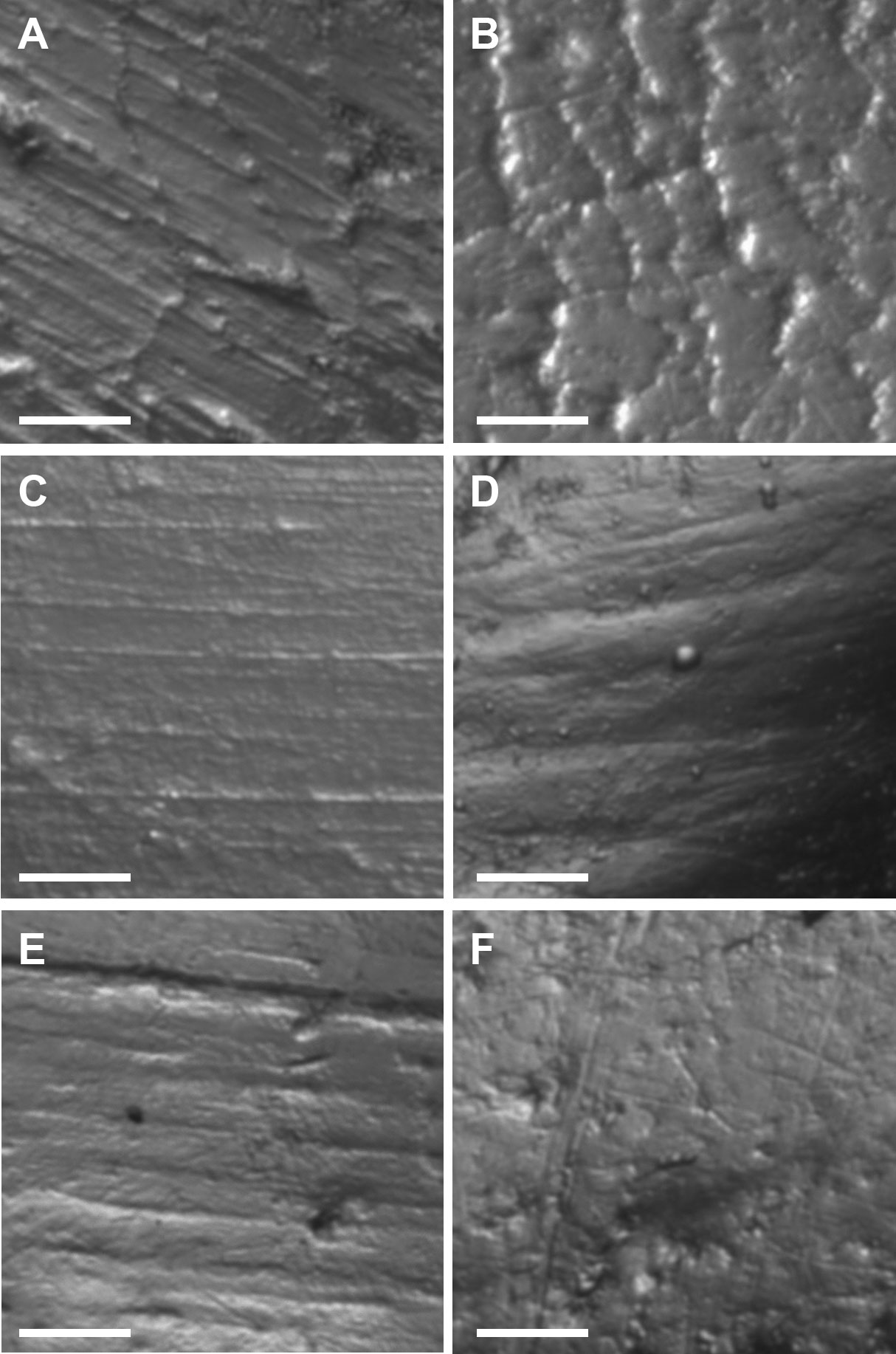
The sample size for tooth microwear, after discarding the specimens showing taphonomic alterations, is as follows: Groβe Ofnet (n = 10), Geißenklösterle (n = 1), Vogelherd AH VII (n = 4), Einhornhöhle (n = 3) and Wolftalhöhle (n = 1). The microwear pattern from the five sites is characterized by relatively low numbers of scratches and pits (Table 1; Fig. 5). In comparison to dietary categories established in extant ungulates, the five samples fall within the dietary space of the extant leaf browsers (Fig. 6). The sample from Groβe Ofnet shows a higher number of scratches (Fig. 5A) than those from the other four localities from the area (Fig. 5C-F). This indicates a diet with greater abrasion for Groβe Ofnet than at the other sites, similar to what was observed in the mesowear analysis. The enamel surfaces also show plucked prisms (Fig. 5B) that are typical in browsing and mixed feeding cervids. The high percentage of individuals with large pits (Table 1) is atypical in leaf browsers and suggests that the giant deer ingested a significant amount of dust or grit together with the vegetation (Semprebon et al. 2011). The absence of hyper-coarse scratches allows us to rule out the consumption of bark from the twigs or branches that could be ingested together with the leaves of woody plants (Semprebon et al. 2011). At the time of death, the giant deer from the five sites had similar diets and were browsing on leaves from trees, shrubs or herbaceous plants and avoiding the woody parts of these plants.
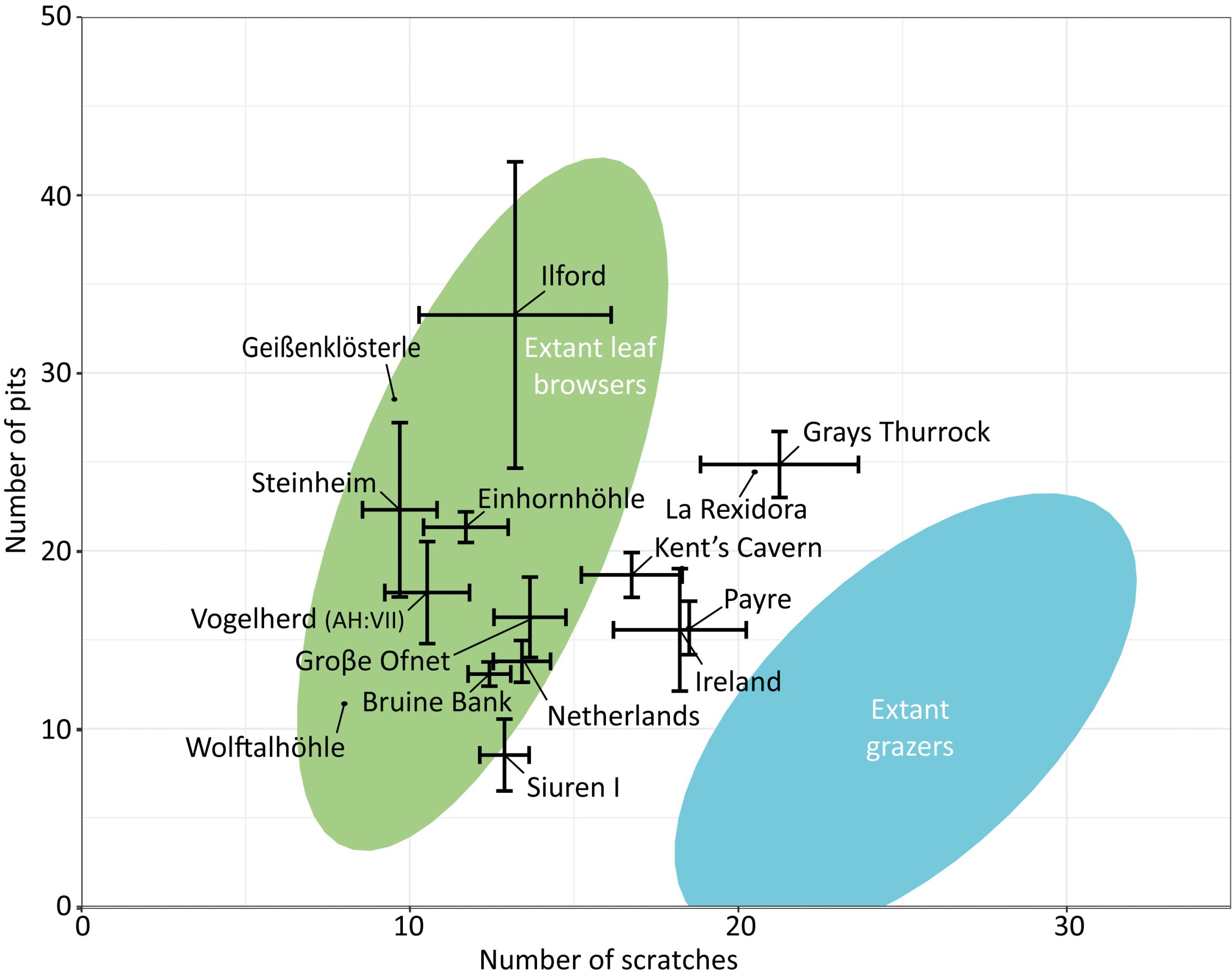
As reported previously in the mesowear analysis, the fossil samples from the other European sites used for comparison show a large range of microwear values. The samples overlap with the extant leaf browsers and mixed feeders, but none of them fall within the ellipse of the pure grazers. There is no apparent temporal or geographical trend related to the age or location of the sites, respectively. This result could be expected because tooth microwear is a short-term signal strongly dependent on the phenology of the plants and thus on seasonality. The seasonal signal may introduce noise into the microwear signal.
Stable carbon, nitrogen and sulfur isotopes from Wolftalhöhle
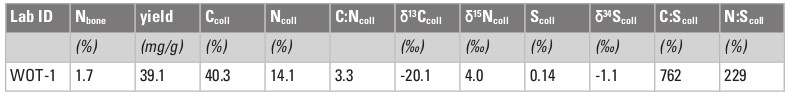
The isotopic values of the giant deer from Wolfthalhöhle are reported in Table 2. The ẟ13C value of –20.1‰ fits with the range of ca. –21 to –18‰ expected for a deer specimen in Europe during the Late Pleistocene (e.g., Immel et al. 2015). The ẟ15N value of +4.0‰ is consistent with those found in large deer in the pre-LGM Swabian Jura (e.g., Münzel et al. 2017). These pre-LGM values are slightly higher than those of the post-LGM deer, including Megaloceros, in the same region, while the animals were re-colonizing territories recently freed from intense permafrost in a warmer and wetter context (Wong et al. 2020). Interestingly, the ẟ34S value (–1.1‰) is comparable to those shown by early Upper Paleolithic reindeer and horses from Geißenklösterle, these relatively low values being typical of the Central European 34S signature during the pre-LGM period (Drucker et al. 2015). All of these results point to an open habitat and are compatible with a mixed-feeding diet.
Discussion and conclusion
The mesowear analysis on the giant deer shows a wide diversity of dietary traits corresponding to food items with different degrees of abrasiveness. The mesowear values of the giant deer from the five assemblages from the Swabian Jura and the other localities in Europe fit with the three main dietary categories of extant herbivores (i.e., leaf browsers, grazers and mixed feeders). Nevertheless, there is a high degree of overlap between the extant mixed feeders and the two other categories, leaf browsers and grazers, and the results should be interpreted in terms of abrasiveness rather than types of diet. Microwear, conversely, shows a better resolution in the discrimination of dietary categories. Considering that microwear did not identify any pure grazer, the diet of the giant deer could be classified as ranging from leaf browsers to grass-dominated mixed feeders. The specific diet observed at each locality depends on the vegetation available regionally and seasonally. The combination of mesowear and microwear analyses allows us to go beyond the simple classification of the giant deer as a mixed feeder and to better characterize the dietary flexibility of that species.
The giant deer from the Swabian Jura and the Harz Mountains show more constrained dietary traits in comparison to the range of diets reported at the scale of Europe. At Groβe Ofnet, the mesowear indicates an average annual diet of grass-dominated mixed feeding, while the microwear suggests a browse-dominated diet at the time of death. The discrepancy between the two proxies could be related to the difference in the timescale between the mesowear and microwear. The giant deer from Große Ofnet were mixed feeders, but died in a season in which it was feeding mainly on browse. At Geißenklösterle, Einhornhöhle, Vogelherd and Wolftalhöhle, the sample size is small, thus limiting the interpretations, but the two proxies provide consistent results for these four localities. The giant deer shows a diet dominated by leaf browsing.
As the five localities analyzed are situated in two different areas (i.e., southern Germany and the Harz Mountains), it is possible to compare the data according to the age of the deposits and the geographic distribution. There is no clear temporal trend between the oldest samples (Einhornhöhle, Wolftalhöhle, Vogelherd AH VII) and the youngest ones (Groβe Ofnet, Geißenklösterle, Vogelherd AH III). Equally, a geographic differentiation is not apparent, which might be a consequence of the small sample from the northern area.
The samples from other localities in Europe show a broad diversity of feeding traits. Some samples show an average annual diet (mesowear) dominated by grass, such as for the giant deer from Ireland, Kent’s Cavern, the Netherlands and Bruine Bank. Others, with lower values, indicate browse-dominated mixed feeding diets, as is the case at Steinheim. The giant deer from Steinheim was assigned to the subspecies M. giganteus antecedens (Berckhemer 1940) and, based on morpho-functional features (e.g., small divergence of antlers: Vislobokova 2013), it was described as a forest-adapted taxa. This assumption made from morphological features is supported by our results revealing a browse-dominated diet. The short-term signal (microwear) is shifted towards leaf browsing and mixed feeding. In the case of the giant deer from the Netherlands and the North Sea, which show similar mesowear and microwear patterns, the mesowear shows a diet dominated by abrasive plants while the microwear indicates that low-abrasion browse was consumed. The plant remains preserved in the molar infundibulum of a giant deer molar from the North Sea were composed of Artemisia (sage) and other Asteraceae, indicating browsing habits in steppe habitats (van Geel et al. 2018).
Previous studies based on morphological features and stable isotopes have also suggested a generalist mixed feeding diet (Aaris Sørensen and Liljegren 2004; Stuart et al. 2004; Chritz et al. 2009). Carbon stable isotope values reflect a feeding strategy based on herbaceous plants and woody browse. In the Swabian Jura, the mesowear and microwear analyses suggest that the giant deer populations were browse-dominated mixed feeders. The stable carbon isotopes from Wolftalhöhle indicate that the giant deer was occupying open landscapes. Similar isotopic results were obtained from younger materials from the Swabian Jura at Hohlenstein Stadel Cave (ca. 14,080 BP; 14,308-13,864 cal BP, ETH-41223) and Hohle Fels Cave (ca. 14,480 BP; 14,82914,194 cal BP, MAMS-16557), also supporting relatively open habitats (Immel et al. 2015). At these two sites, there is no dental material available for dental wear analyses. Conversely, at older sites from southern Germany, Steinheim (MIS 11) and Villa Seckendorff (MIS 5 a-d), the relatively more negative stable carbon isotope values suggest that the giant deer inhabited a forested landscape (dense forest to sparse woodland formation) (Pushkina et al. 2020). For Steinheim, this result is also supported by mesowear and microwear, which indicate a diet dominated by browse (Rivals and Ziegler 2018; Pushkina et al. 2020).
Giant deer populations from the Swabian Jura inhabited relatively open habitats, probably with boreal parkland vegetation, and their diet reflects preferences for leaf browsing and mixed feeding. At the scale of Europe, the giant deer had a flexible diet and was probably able to feed on both browse and grass. This permitted the giant deer to adapt to major environmental changes and to survive throughout the Pleistocene. However, at the end of the Pleistocene and during the early Holocene, giant deer were not abundant, and populations were fragmented (Lister and Stuart 2019). Depending on the region, the giant deer suffered from habitat loss due to the change of the boreal parkland to tundra-like habitats in northwest Europe or to closed woodlands in central Eurasia. By this time, the giant deer populations could not cope with the drastic climatic and vegetational changes; this led to the giant deer’s extirpation (Lister and Stuart 2019). In southern Germany, competition with other cervids due to overlapping niches was proposed to be part of the cause of extinction (Immel et al. 2015). The results of the mesoand microwear analyses allowed us to identify a certain degree of dietary plasticity for the giant deer. The diet itself is probably not related to the giant deer’s extinction, and other causes, such as intolerance to the density of the forest cover, competition with other cervids and/or the spread of human populations, could have contributed to its extirpation.
Acknowledgements
We thank Àngel Blanco-Lapaz for his help in sampling and molding the specimens and preparing the maps and the pictures of the specimens. We are grateful to Thomas Rathgeber, Thomas Terberger, Thorsten Uthmeier, Reinhard Ziegler for their help in accessing the collections. FR was supported by the SYNTHESYS Project financed by European Community Research Infrastructure Action under the FP7 Integrating Activities Programme (grant DE-TAF-5375) to visit the Staatliches Museum für Naturkunde in Stuttgart for sampling the Groβe Ofnet specimens. FR research is supported by the Spanish Ministry of Science and Innovation through the projects PID2019-103987GB-C31 and the “María de Maeztu” excellence accreditation (CEX2019000945-M), by the Generalitat de Catalunya and AGAUR projects CLT009/18/00054, CLT009/18/00055 and 2017-SGR-836. Finally, we thank Michael Bolus and two anonymous reviewers for their help in improving this work.
References
Aaris-Sørensen, K. and Liljegren, R. 2004: Late Pleistocene Remains of Giant Deer (Megaloceros giganteus Blumenbach) in Scandinavia: Chronology and Environment. Boreas 33, 61–73.
Allen, J. R. M., Hickler, T., Singarayer, J. S., Sykes, M. T., Valdes, P. J., and Huntley, B. 2010: Last Glacial Vegetation of Northern Eurasia. Quaternary Science Reviews 29, 2604–2618.
Berckhemer, F. 1940: Über die Riesenhirschfunde von Steinheim an der Murr. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg 96, 63–88.
Bocherens, H. 2003: Isotopic Biogeochemistry and the Paleoecology of the Mammoth Steppe Fauna. Deinsea 9, 57–76.
Bocherens, H., Fizet, M., Mariotti, A., Lange-Badre, B., Vandermeersch, B., Borel, J. P., and Bellon, G. 1991: Isotopic Biogeochemistry (13C, 15N) of Fossil Vertebrate Collagen: Application to the Study of a Past Food Web Including Neandertal Man. Journal of Human Evolution 20, 481–492.
Chritz, K. L., Dyke, G. J., Zazzo, A., Lister, A. M., Monaghan, N. T., and Sigwart, J. D. 2009: Palaeobiology of an Extinct Ice Age Mammal: Stable Isotope and Cementum Analysis of Giant Deer Teeth. Palaeogeography, Palaeoclimatology, Palaeoecology 282, 133–144.
Conard, N. J., Bolus, M., Dutkiewicz, E. and Wolf, S. 2015: Eiszeitarchäologie auf der Schwäbischen Alb. Die Fundstellen im Ach- und Lonetal und in ihrer Umgebung. Tübingen: Kerns Verlag.
Conard, N. J., Zeidi, M., and Janas, A. 2016: Abschließender Bericht über die Nachgrabung am Vogelherd und die Sondage in der Wolftalhöhle. Archäologische Ausgrabungen in Baden-Württemberg 2015, 66–72.
Conard, N. J., Bolus, M. and Münzel, S. C. (eds.) 2019: Geißenklösterle. Chronostratigraphie, Paläoumwelt und Subsistenz im Mittel- und Jungpaläolithikum der Schwäbischen Alb. Tübingen: Kerns Verlag.
Croitor, R., Sanz, M., and Daura, J. 2020: The endemic deer Haploidoceros mediterraneus (Bonifay) (Cervidae, Mammalia) from the Late Pleistocene of Cova del Rinoceront (Iberian Peninsula): Origin, Ecomorphology, and Paleobiology. Historical Biology 32, 409–427.
DeNiro, M. J. 1985: Postmortem Preservation and Alteration of in vivo Bone Collagen Isotope Ratios in Relation to Palaeodietary Reconstruction. Nature 317, 806–809.
Drucker, D. G., Vercoutère, C., Chiotti, L., Nespoulet, R., Crépin, L., Conard, N. J., Münzel, S. C., Higham, T., van der Plicht, J., Lázničková-Galetová, M., and Bocherens, H. 2015: Tracking Possible Decline of Woolly Mammoth During the Gravettian in Dordogne (France) and the Ach Valley (Germany) Using Multi-Isotope Tracking (13C, 14C, 15N, 34S, 18O). Quaternary International 359-360, 304–317.
Fortelius, M. and Solounias, N. 2000: Functional Characterization of Ungulate Molars Using the Abrasion-Attrition Wear Gradient: A New Method for Reconstructing Paleodiets. American Museum Novitates 3301.
Hahn, J. 1988: Die Geißenklösterle-Höhle im Achtal bei Blaubeuren I. Fundhorizontbildung und Besiedlung im Mittelpaläolithikum und im Aurignacien. Stuttgart: Konrad Theiss Verlag.
Higham, T., Basell, L., Jacobi, R., Wood, R., Bronk Ramsey, C., and Conard, N. J. 2012: Τesting Models for the Beginnings of the Aurignacian and the Advent of Figurative Art and Music: The Radiocarbon Chronology of Geißenklösterle. Journal of Human Evolution 62, 664–676.
Immel, A., Drucker, D. G., Bonazzi, M., Jahnke, T. K., Münzel, S. C., Schuenemann, V. J., Herbig, A., Kind, C.-J., and Krause, J. 2015: Mitochondrial Genomes of Giant Deers Suggest their Late Survival in Central Europe. Scientific Reports 5: 10853.
Kahlke, R. D. 1999: The History of the Origin, Evolution and Dispersal of the Late Pleistocene Mammuthus-Coelodonta Faunal Complex in Eurasia (Large Mammals). Rapid City: Fenske Companies.
Kaufmann, G., Romanov, D., Nielbock, R., and Lundberg, J. 2020: The Sediment Record of the Unicorn Cave, Southern Harz Mountains, Germany. Geomorphology 367: 107295.
King, T., Andrews, P., and Boz, B. 1999: Effect of Taphonomic Processes on Dental Microwear. American Journal of Physical Anthropology 108, 359–373.
Koken, E. 1912: Die Geologie und Tierwelt der paläolithischen Kulturstätten Deutschlands. In: R. R. Schmidt, Die diluviale Vorzeit Deutschlands. Stuttgart: E. Schweizerbartsche Verlagsbuchhandlung Nägele und Dr. Sproesser, 159–228.
Kotula, A., Leder, D., Lehmann, J., Hillgruber, K. F., Nielbock, R., and Terberger, T. 2019: Eiszeitliche Besiedlung in Niedersachsens Höhlen – neue Forschungen an der Einhornhöhle im Harz, Ldkr. Göttingen. Nachrichten aus Niedersachsens Urgeschichte 88, 213–231.
Leder, D., Hermann, R., Hüls, M., Russo, G., Hoelzmann, P., Nielbock, R., Böhner, U., Lehmann, J., Meier, M., Schwalb, A., Tröller-Reimer, A., Koddenberg, T., and Terberger, T. 2021: A 51,000-Year-Old Engraved Bone Reveals Neanderthals’ Capacity for Symbolic Behaviour. Nature Ecology & Evolution 5, 1273–1282.
Lister, A. M. 1981: Evolutionary Studies on Pleistocene Deer. Unpublished PhD dissertation, University of Cambridge.
Lister, A. M. 1994: The Evolution of the Giant Deer, Megaloceros giganteus (Blumenbach). Zoological Journal of the Linnean Society 112, 65–100.
Lister, A. M. and Stuart, A. J. 2019: The Extinction of the Giant Deer Megaloceros giganteus (Blumenbach): New Radiocarbon Evidence. Quaternary International 500, 185–203.
Mihlbachler, M. C., Rivals, F., Solounias, N., and Semprebon, G. M. 2011: Dietary Change and Evolution of Horses in North America. Science 331, 1178–1181.
Moreau, L. 2009: Geißenklösterle: Das Gravettien der Schwäbischen Alb im europäischen Kontext. Tübingen: Kerns Verlag.
Münzel, S. C., Wolf, S., Drucker, D. G., and Conard, N. J. 2017: The Exploitation of Mammoth in the Swabian Jura (SWGermany) During the Aurignacian and Gravettian Period. Quaternary International 445, 184–199.
Nehlich, O. and Richards, M. P. 2009: Establishing Collagen Quality Criteria for Sulphur Isotope Analysis of Archaeological Bone Collagen. Archaeological and Anthropological Sciences 1, 59–75.
Niven, L. 2006: The Palaeolithic Occupation of Vogelherd Cave. Implications for the Subsistence Behavior of Late Neanderthals and Early Modern Humans. Tübingen: Kerns Verlag.
Pushkina, D. and Raia, P. 2008: Human Influence on Distribution and Extinctions of the Late Pleistocene Eurasian Megafauna. Journal of Human Evolution 54, 769–782.
Pushkina, D., Saarinen, J., Ziegler, R., and Bocherens, H. 2020: Stable Isotopic and Mesowear Reconstructions of Paleodiet and Habitat of the Middle and Late Pleistocene Mammals in South-Western Germany. Quaternary Science Reviews 227: 106026.
Ramírez-Pedraza, I., Rivals, F., Uthmeier, T., and Chabai, V. 2020: Palaeoenvironmental and Seasonal Context of the Late Middle and Early Upper Palaeolithic Occupations in Crimea: An Approach Using Dental Wear Patterns in Ungulates. Archaeological and Anthropological Sciences 12: 268.
Richard, M., Falguères, C., Valladas, H., Ghaleb, B., Pons-Branchu, E., Mercier, N., Richter, D., and Conard, N. J. 2019: New Electron Spin Resonance (ESR) Ages from Geißenklösterle Cave: A Chronological Study of the Middle and Early Upper Paleolithic Layers. Journal of Human Evolution 133,133-145.
Richards, M. P., Pettitt, P. B., Stiner, M. C., and Trinkaus, E. 2001: Stable Isotope Evidence for Increasing Dietary Breadth in the European mid-Upper Paleolithic. Proceedings of the National Academy of Sciences of the U.S.A. 98, 6528–6532.
Riek, G. 1934: Die Eiszeitjägerstation am Vogelherd im Lonetal I: Die Kulturen. Tübingen: Akademische Buchhandlung Franz F. Heine.
Rivals, F. and Álvarez-Lao, D. J. 2018: Ungulate Dietary Traits and Plasticity in Zones of Ecological Transition Inferred from Late Pleistocene Assemblages at Jou Puerta and Rexidora in the Cantabrian Region of Northern Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 499, 123–130.
Rivals, F. and Lister, A. M. 2016: Dietary Flexibility and Niche Partitioning of Large Herbivores Through the Pleistocene of Britain. Quaternary Science Reviews 146, 116–133.
Rivals, F. and Ziegler, R. 2018: High-Resolution Paleoenvironmental Context for Human Occupations During the Middle Pleistocene in Europe (MIS 11, Germany). Quaternary Science Reviews 188, 136–142.
Rivals, F., Mihlbachler, M. C., and Solounias, N. 2007: Effect of Ontogenetic-Age Distribution in Fossil and Modern Samples on the Interpretation of Ungulate Paleodiets Using the Mesowear Method. Journal of Vertebrate Paleontology 27, 763–767.
Rivals, F., Schulz, E., and Kaiser, T. M. 2009: Late and Middle Pleistocene Ungulates Dietary Diversity in Western Europe Indicate Variations of Neanderthal Paleoenvironments Through Time and Space. Quaternary Science Reviews 28, 3388–3400.
Rivals, F., Mihlbachler, M. C., Solounias, N., Mol, D., Semprebon, G. M., de Vos, J., and Kalthoff, D. C. 2010: Palaeoecology of the Mammoth Steppe Fauna from the late Pleistocene of the North Sea and Alaska: Separating Species Preferences from Geographic Influence in Paleoecological Dental Wear Analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 286, 42–54.
Rivals, F., Rindel, D., and Belardi, J. B. 2013: Dietary Ecology of Extant Guanaco (Lama guanicoe) from Southern Patagonia: Seasonal Leaf Browsing and Its Archaeological implications. Journal of Archaeological Science 40, 2971–2980.
Rivals, F., Takatsuki, S., Albert, R. M., and Macià, L. 2014: Bamboo Feeding and Tooth Wear of Three Sika Deer (Cervus nippon) Populations from Northern Japan. Journal of Mammalogy 95, 1043–1053.
Schmidt, R. R. 1912: Die diluviale Vorzeit Deutschlands. With contributions by E. Koken and A. Schliz. Stuttgart: E. Schweizerbartsche Verlagsbuchhandlung Nägele und Dr. Sproesser.
Semprebon, G. M., Godfrey, L. R., Solounias, N., Sutherland, M. R., and Jungers, W. L. 2004: Can Low-Magnification Stereomicroscopy Reveal Diet? Journal of Human Evolution 47, 115–144.
Semprebon, G. M., Sise, P. J., and Coombs, M. C. 2011: Potential Bark and Fruit Browsing as Revealed by Stereomicrowear Analysis of the Peculiar Clawed Herbivores known as Chalicotheres (Perissodactyla, Chalicotherioidea). Journal of Mammalian Evolution 18, 33–55.
Solounias, N. and Semprebon, G. 2002: Advances in the Reconstruction of Ungulate Ecomorphology with Application to Early Fossil Equids. American Museum Novitates 3366.
Stuart, A. J., Kosintsev, P. A., Higham, T. F. G. and Lister, A. M. 2004: Pleistocene to Holocene Extinction Dynamics in Giant Deer and Woolly Mammoth. Nature 431, 684–689.
Uthmeier, T. 2004: Micoquien, Aurignacien und Gravettien in Bayern Eine regionale Studie zum Übergang vom Mittel- zum Jungpaläolithikum. Archäologische Berichte 18. Bonn: Rudolf Habelt.
van Geel, B., Sevink, J., Mol, D., Langeveld, B. W., van der Ham, R. W. J. M., van der Kraan, C. J. M., van der Plicht, J., Haile, J. S., Rey-Iglesia, A., and Lorenzen, E. D., 2018: Giant Deer (Megaloceros giganteus) Diet from Mid-Weichselian Deposits under the Present North Sea Inferred from Molar-Embedded Botanical Remains. Journal of Quaternary Science 33, 924–933.
Vislobokova, I. A. 2012: Giant Deer: Origin, Evolution, Role in the Biosphere. Paleontological Journal 46, 643–775.
Vislobokova, I. A. 2013: Morphology, Taxonomy, and Phylogeny of Megacerines (Megacerini, Cervidae, Artiodactyla). Paleontological Journal 47, 833–950.
Wong, G. L., Starkovich, B. M., Drucker, D. G., and Conard, N. J., 2020: New Perspectives on Human Subsistence During the Magdalenian in the Swabian Jura, Germany. Archaeological and Anthropological Sciences 12: 217.