Abstract
In recent years, new evidence for the early use of plant foods has challenged the stereotype of the meat-eating Paleolithic. Whilst often making up the smaller component of the diet, plant foods are key to hominin diets, carbohydrates especially providing an efficient energy resource. This paper reviews the current evidence for the role of plant foods in the evolution and dispersal of early modern humans and our closest ancestors, with a focus on new evidence for early diet from Island Southeast Asia, Australia and New Guinea. It demonstrates the importance of plant foods and their processing, to the dietary flexibility and adaptive capacity of our species.
Introduction
As a species, Homo sapiens have thrived beyond the extinction of our closest hominin ancestors and have adapted to many and diverse environments, using cultural innovations to not only survive within but to shape the world around us. Diet, and the ability to extract energy and nutrition from diverse resources and ecological niches, has long been seen as key to this process of evolution and dispersal. However, research into Paleolithic diets has historically focused on the animal component: meat and its associated hunting behaviors and technologies. Whilst this focus reflects the biases of the archaeological record, it has also been broadly supported by an assertion that most calories in modern hunter-gatherer (Cordain et al. 2000; cf. Milton 2000) and—where isotopic analysis has offered an insight—Paleolithic diets are derived from animal sources (Richards et al. 2000; Bocherens et al. 2005; Bocherens 2009; Richards and Trinkaus 2009; Wißing et al. 2016; Jaouen et al. 2019; cf. Naito et al. 2016; Drucker et al. 2017). However, more recent research has revisited the importance of plant foods to Paleolithic diets. This has been a result of new evidence and, in many cases, novel methods (e.g., analysis of plant microfossils in dental calculus). It has also been a result of a shift in focus to understand the macronutrient requirements of hominin populations, and the role of hard-to-process and non-preferred or ‘fallback’ foods, including many plant foods (Robinson and Wilson 1998; Laden and Wrangham 2005), in their evolution and dispersal. This has led to new considerations of the importance of plant foods in the movement of archaic and modern humans into more marginal environments (Jones 2009; Hardy 2010), and of the ultimate success of modern human populations (Stiner and Kuhn 2009; Power and L’Engle Williams 2018).
Whilst much of this debate has been centered on Africa and Eurasia, the archaeology of Island Southeast Asia, Australia and New Guinea has in recent years provided new evidence for the role of plant foods in early adaptations to novel environments (see Fig. 1; Barton 2005; Barker et al. 2007; Summerhayes et al. 2010; Dilkes-Hall et al. 2019; Florin et al. 2020). The movement of humans across the Wallacean Archipelago and the peopling of Sahul (the Pleistocene continent encompassing Australia, New Guinea and the Aru Islands) represents a critical threshold in human history, human populations crossing a series of biogeographic boundaries impassable for all kinds of animals, including several of our hominin ancestors (H. floresiensis; Brown et al. 2004; Sutikna et al. 2016; H. luzonensis; Détroit et al. 2019; and, possibly, Denisovans; Carlhoff et al. 2021; Teixeira et al. 2021). This paper reviews the current evidence for the role of plant foods in the evolution and dispersal of modern humans and our closest ancestors, and uses evidence from the southern dispersal arc to provide new perspectives on the importance of plant gathering, cooking and processing to the adaptive capacity of humans across a range of environments.
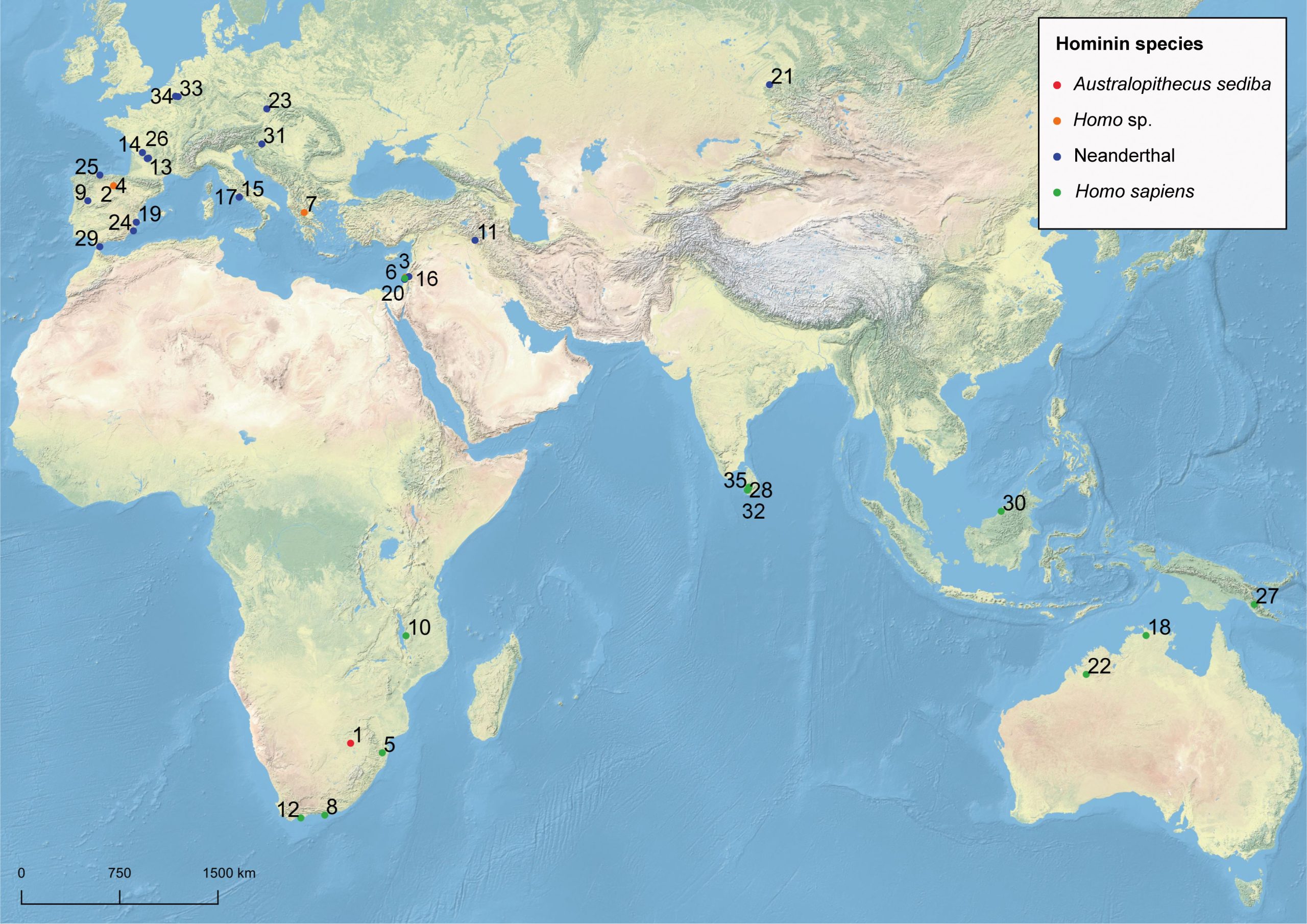
1: Malapa, South Africa; 2: Sima del Elefante, Spain; 3: Gesher Benot Ya’aqov, Israel; 4: Gran Dolina, Spain; 5: Border Cave, South Africa; 6: Skhul Cave, Israel; 7: Theopetra Cave, Greece; 8: Klasies River Caves, South Africa; 9: Figueira Brava Cave, Portugal; 10: Ngalue Cave, Mozambique; 11: Shanidar Cave, Iran; 12: Blombos Cave, South Africa; 13: La Ferrassie I and II, France; 14: La Quina, France; 15: Grotta del Fossellone, Italy; 16: Amud Cave, Israel; 17: Grotta Guattari, Italy; 18: Madjedbebe, Australia; 19: El Salt, Spain; 20: Kebara Cave, Israel; 21: Chagyrskaya Cave, Siberia; 22: Carpenter’s Gap 1, Australia; 23: Kůlna Cave, Czech Republic; 24: Sima de las Palomas del Cabezo Gordo, Spain; 25: El Sidrón, Spain; 26: Abris des Merveilles, France; Ivane Valley, New Guinea; 28: Fa-hien Lena, Sri Lanka; 29: Gorham’s Cave, Gibraltar; 30: Niah Cave, Borneo; 31: Vindija Cave, Croatia; 32: Kitulgala Beli-lena, Sri Lanka; 33: Goyet Caves, Belgium; 34: Spy Cave, Belgium; 35: Batadomba-lena, Sri Lanka; ESRI base map.
Diet, and hominin evolution and dispersal in Africa and Eurasia
Diet has long been considered important to hominin evolution. Many of the physiological traits unique to the Homo genus, including our relatively large brain size, decreased gut size, and changes in dental morphology and locomotive adaptations suggest innovative dietary choices comparative to other primates. However, the evidence available to directly consider the diet of different hominin species is scarce, especially in earlier time periods, and tends to favor evidence for the more robust, animal component of diet. Where the remains of plant foods do preserve, it is often as charred macrofossils—the processes of cooking, burning fuel or disposing of rubbish in hearths allowing for continued preservation of these materials through carbonization; as more robust fossil remains (e.g., phytoliths, mineralized seeds); or in special preservational environments (e.g., water-logged sites, within dental calculus). However, with increased antiquity, the recovery of plant food remains requires both exceptional preservational environments and the implementation of systematic archaeobotanical recovery techniques. Therefore, a range of indirect evidence, including changes in phenotypic traits. and genetics, and comparisons to contemporary human and nonhuman primate populations are often required, alongside archaeological evidence, to build a picture of evolving hominin diet.
For the majority of hominin evolution diet has been heavily plant-based, and early australopiths likely ate a diet similar to that of their closest ancestors, great apes. This meant a diet heavily focused on fruits, with leaves, flowers and stem consumed as secondary or fallback foods (Doran et al. 2002; Rothman et al. 2008; Hohmann et al. 2010; Watts et al. 2012; Vogel et al. 2017). All great apes also consume some insect matter, which provide trace nutrients. Chimpanzees and bonobos, especially dominant males, hunt and kill small vertebrates and monkeys, which can make up to 10-20% of their diet (Milton 1987; Watts 2020). However, the majority of chimpanzees and bonobos, and likely early hominins, acquire the substantive proportion of their calories from fruit (~60-70%) and leaves (20%; Pontzer and Wood 2021). Changes in the microwear and dental morphology of Australopithecus, and to a greater degree Paranthropus, including increases in molar size, enamel thickness and decreases in shearing quotients, has been suggested to indicate a shift to a broader plant-based diet, with focus potentially shifting from leaves to underground storage organs (USOs) as a fallback food 4-2 million years ago (mya; Ungar 2004; Laden and Wrangham 2005). This is supported by isotopic evidence for C4 resource consumption by A. bahrelghazali individuals from Koro Toro in Chad, which is argued to demonstrate the exploitation of the above- and below-ground parts of grasses (Poaceae) and sedges (Cyperaceae), especially sedge tubers and corms (Lee-Thorp et al. 2012).
Meat-eating
More than 2.5 mya (Bunn 1981; Potts and Shipman 1981; Domínguez-Rodrigo et al. 2005), and possibly as early as 3.3 mya (McPherron et al. 2010), cut marks found on fossil faunal assemblages attest to the hunting and confrontational scavenging of large herbivores by African hominids (Domínguez-Rodrigo and Pickering 2003; cf. O‘Connell et al. 2002). This increase in meat-eating associated with late Australopithecus and early Homo, broadly corresponds to the evolution of a significantly larger relative brain size, increased body size, simplified digestive anatomy, and greatly expanded geographical range occurring with H. erectus by 1.8 mya (Wood and Collard 1999a, b). As such, increased carnivory has been argued to be a central mechanism in the evolutionary development of Homo (Aiello and Wheeler 1995; Milton 1999; Bunn 2007; Zink and Lieberman 2016).
Aiello and Wheeler’s (1995) ‘expensive-tissue hypothesis’ argues that the energy expenses of relative increases in brain size in Homo were offset by decreasing gut size and, therefore, required a high-quality, easy-to-digest diet. The addition of hunting into the Hominin behavioral repertoire is argued to have provided such a high-calorie, low-fiber resource (Bunn 2007), and evidence of changing masticatory capacity in Homo, including decreased enamel thickness and increases in the shearing quotients compared to Australopithecus, supports the inclusion of more, tough and elastic resources, such as meat, into early Pleistocene diets (Ungar 2004). However, whilst meat is high-calorie and provides easily digestible essential amino acids, minerals and vitamins, the metabolic cost of converting protein into energy, required for brain function and the physical demands of hunting (e.g., endurance running; Bramble and Lieberman 2004), greatly exceeds that of carbohydrates (Milton 1999). Meat-eating has usually been argued, therefore, to have been incorporated into a mixed diet, potentially also allowing for a shift towards the consumption of a higher-proportion of energy-rich, rather than nutrient-rich, plant resources, such as seeds, nuts and USOs (Milton 1999, 2003). Early direct evidence for plant food use is sparse. However, there is evidence for the use of uncooked starchy plant foods and nutcracking, dating to ~1.2 mya and 790 kya, respectively (see Table 1; Goren-Inbar et al. 2002; Hardy et al. 2017).

 Table 1: Direct evidence for the use of plant foods, dating to >35 kya; †Homo sapiens also occupied the site, but not during this time period; *Celtis spp. endocarp have also been found at several other Early and Middle Pleistocene archaeological sites (Chaney 1935; Aigner 1969; de Lumley et al. 1976; Laville and Renault-Miskovsky 1977; Binford and Ho 1985; Matsutani 1987; Bittman 1992; Gabunia et al. 2000; Messager et al. 2008). However, these assemblages have been largely interpreted as environmental in origin.
Table 1: Direct evidence for the use of plant foods, dating to >35 kya; †Homo sapiens also occupied the site, but not during this time period; *Celtis spp. endocarp have also been found at several other Early and Middle Pleistocene archaeological sites (Chaney 1935; Aigner 1969; de Lumley et al. 1976; Laville and Renault-Miskovsky 1977; Binford and Ho 1985; Matsutani 1987; Bittman 1992; Gabunia et al. 2000; Messager et al. 2008). However, these assemblages have been largely interpreted as environmental in origin.Cooking and starchy plant foods
Changes in dietary composition are not the only way to improve dietary quality. The processing of foods, especially cooking, has also been implicated in the evolution of increased hominin brain size (Wrangham et al. 1999; Wrangham 2007; 2017; Wrangham and Carmody 2010). Wrangham et al.’s ‘cooking hypothesis’ argues that the ability to cook food, rather than meateating, was the likely driver for the encephalization of our species ~2 mya. This is because cooking makes many foods easier to digest, especially starchy plant foods (e.g., USOs, grass seeds) through the conversion of resistant starches to soluble forms; kills foodborne pathogens; and necessitates the assembly of foods into a shared location, heightening competition and facilitating new social relationships (Wrangham et al. 1999; Carmody and Wrangham 2009; see also O’Connell et al. 1999). There is, however, no archaeological evidence for the use of fire by hominins prior to ~1.5–1 mya (Brain and Sillen 1988; Bellomo 1994; Berna et al. 2012), and repeated and consistent fire use is not apparent until 400–200 thousand years ago (kya; Roebroeks and Villa 2011; Shahack-Gross et al. 2014; Sandgathe 2017; Sorensen 2019). It is, therefore, possible that cooking was not a key driver of earlier hominin evolution, including physiological and behavioral changes evident in early H. erectus. However, its effect on modern human physiology is readily apparent, as, even with access to domesticates and electric blenders, the contemporary choice to subsist on uncooked plant foods has been shown to cause undernutrition, reduced libido, amenorrhea and fatigue (Koebnick et al. 1999).
One hypothesized effect of cooking and other plant-processing practices on human physiology is copy number expansion (increased repetition of the relevant section of the genome) in the αamylase gene responsible for the production of salivary amylase, AMY1 (Hardy et al. 2015; Inchley et al. 2016). α-amylase is an enzyme responsible for catalyzing the hydrolysis of starch into sugars, and is produced in the pancreas and salivary glands of humans. Whilst the role of salivary, as opposed to pancreatic, amylase in starch breakdown is still somewhat unclear (Sonestedt 2018), increased salivary amylase has been associated with changes in the oral perception of starches, leading to increased palatability, and the body’s pre-absorptive metabolic signalling, allowing for more effective digestion (Hoebler et al. 1998; Mandel et al. 2010; Mandel and Breslin 2012; Peyrot des Gachons and Breslin 2016; Atkinson et al. 2018). Further, high copy numbers of AMY1 (up to 30) and associated increased salivary amylase production, correlate with the consumption of high-starch diets both within contemporary human populations, and between humans and non-human primates, the latter only possessing two copy numbers (Samuelson et al. 1996; Perry et al. 2007). A recent study investigating the AMY gene locus in ancient and modern DNA found that a copy number expansion occurred in AMY1 following the split between Neanderthals and modern humans ~650 kya and prior to the movement of humans outside Africa (Inchley et al. 2016). Inchley et al. (2016) argue this copy number expansion occurred in a selective environment in which increased starch consumption favored changes in starchdigestion efficiency. This increased starch consumption was, they argue, facilitated by the development of plant processing practices in the Middle Pleistocene, such as cooking, grinding, and leaching.
Whilst Neanderthals only possess two AMY1 copy numbers (Prüfer et al. 2014), a recent genomic analysis of ancient and modern oral microbiomes of Homo and nonhuman primates, provided evidence for abundant oral Streptococci bacteria in both modern humans and Neanderthals, but not in nonhuman primates (Fellows Yates et al. 2021). These bacteria possess amylase-binding protein genes, which when expressed capture salivary amylase, for their nutrition and dental adhesion. This suggests starch consumption had begun to increase prior to the split between Neanderthals and modern humans. Later copy number variations in AMY1 likely reflected a growing trend in the increasing use and processing of starchy plant foods, potentially with roots in the pre-Homo dietary incorporation of USOs 2-4 mya.
This is supported by a sparse, but growing body of direct evidence for the use, cooking and processing of starchy plant foods by both modern humans and Neanderthals in Africa and Eurasia (see Table 1). This includes macrobotanical evidence for the cooking of USOs by modern humans by 170 kya in South Africa (Larbey et al. 2019; Wadley et al. 2020); and diagnostic processing damage on starch grains recovered from both modern human and Neanderthal dental calculus, 130-100 kya and 50-46 kya, respectively, in Israel, suggesting the cooking and eating of starchy plant foods (Henry et al. 2011, 2014). Wild grass starch grains with processing damage consistent with grinding, recovered from the surfaces of stone tools, such as scrapers and core/grinding tools, at Ngalue Cave in Mozambique also attest to seed-grinding by modern humans by 105 kya (Mercader 2009).
Hyper-carnivorism and animal fat
However, this evidence does not allow for easy interpretations of the proportional contributions of plant and animal foods to diet, and some researchers still argue for a heavy reliance on large animal foods by Middle Pleistocene Homo in Africa and Eurasia (Ben-Dor et al. 2011, 2021; Ben-Dor and Barkai 2021). The ‘hyper-carnivore hypothesis’ argues H. erectus and later Middle Paleolithic hominins (including Neanderthals and early modern humans) relied on animal foods for over 70% of their caloric intake. To achieve this, they required high animal fat contributions to their diet, to offset protein consumption. This is because protein consumption has a physiological limit in humans, placed at ~20-50% of daily calorie intake (Billsborough and Mann 2006; Speth and Spielmann 1983; Noli and Avery 1988). This limit is due both to the high metabolic cost of processing protein for energy, which raises daily caloric requirements, and the limited ability of the liver and kidneys to remove the by-products of protein metabolism, especially nitrogen-containing urea (Billsborough and Mann 2006; Rudman et al. 1973). The documented effects of eating a diet based primarily in lean protein, known as ‘rabbit starvation’, are starvation despite high-calorie consumption, loss of body protein, protein toxicity and, after several weeks, death (Stefansson 1944; Speth and Spielmann 1983). Both fats and carbohydrates spare the metabolism of protein for energy and their consumption is required to maintain a high-protein intake. Ben-Dor and colleagues (Ben-Dor et al. 2011, 2021; Ben-Dor and Barkai 2021) hypothesize that a specialization in large prey, epitomized by the hunting of elephants, by Middle Pleistocene Homo allowed them to consume the high-fat diet required to live as hyper-carnivores. Decreases in prey-size, due to a mixture of over-exploitation and changing environments, they argue, led to decreased fat-intake, a subsequent broadening of diet to offset this, and increasingly complex behavioral and cultural adaptations (Ben-Dor and Barkai 2021).
However, numerous studies have shown that under conditions of marginal calorie, protein or glucose intake, carbohydrates spare protein more efficiently than fat, making plant foods an often more efficient substitute to fatty animals despite their higher processing requirements (Speth and Spielmann 1983). Further, modern examples of hyper-carnivorism—such as arcticliving, where a high-fat intake is met by blubber-rich marine mammals, or pastoralism, which relies on domestication and secondary products for fat and protein consumption—are recent adaptions to marginal environments (Vasil‘ev et al. 2016; Arbuckle and Hammer 2018; Flegontov et al. 2019; Pontzer and Wood 2021). Wild animals from sub-arctic environments typically have much lower levels of body fats than those involved in these examples (<5%; Cordain et al. 2000; Hardy 2010). Hyper-carnivorism, therefore, demonstrates the extreme flexibility of the human dietary niche. However, it is very unlikely, even given the higher fat content of larger extinct Pleistocene mammals (Ben-Dor and Barkai 2021), that it was a preferred diet across Middle Pleistocene Africa and Eurasia, especially in warmer tropical and temperate regions. Indeed, contemporary hunter-gatherer groups from temperate and tropical regions depend on gathered plant foods for at least ~26-55% of their calories (Cordain et al. 2000), and it is argued that the risk involved in large game hunting in many of these societies means it is practiced as much for its benefits as a competitive male display and its role in the production of reciprocal relationships through meat-sharing, as it is for its nutritional advantages (Hawkes et al. 1991; O’Connell et al. 2002). It is only in the northern coniferous forest and tundra that plant foods make up less than 25% of hunter-gatherer calories (Cordain et al. 2000; cf. Milton 2000).
The dietary breadth of Neanderthals and modern humans in Europe
In the glacial, high-altitude environments of Middle Paleolithic Europe, it is, therefore, not surprising that there is both zooarchaeological and isotopic evidence to suggest that Neanderthal populations may have relied heavily on the hunting of wooly mammoths and large ungulates, such as gazelle, deer, wild horses, boar, bison, and wild cattle (Hoffecker 1991; Stiner 1994; Gaudzinski 1995; Thieme 1997; Marean and Assefa 1999; Burke 2000; Richards et al. 2000; Griggo 2004; Bocherens et al. 2005; Kuhn and Stiner 2006; Bocherens 2009; Richards and Trinkaus 2009; Stiner and Kuhn 2009; Wißing et al. 2016; Jaouen et al. 2019). Kuhn and Stiner (Kuhn and Stiner 2006; Stiner and Kuhn 2009) have argued the archaeological signature of this dietary pattern is markedly different to that of Upper Paleolithic modern human populations in Europe, who were increasingly more reliant on small hard-to-capture prey, aquatic resources and plant carbohydrates evidenced by the increased frequency of grinding stone technologies in archaeological sites. They link this dietary shift, to other technological changes in the Upper Paleolithic, including the development of bone needles and awls required for the production of clothing and shelter, which are associated with gendered division of labor ethnographically. As such, they argue, Upper Paleolithic populations broadened their dietary breadth through the development of complimentary, gendered economic roles, comparable to those of most modern hunter-gatherer economies. The adaptive advantages of specialized division of labor, namely increased dietary breadth, rather than a specific difference in cognitive or physiological capabilities, is argued to have allowed the demographic expansion of modern human populations and, eventual, outcompetition of contemporary Neanderthal populations in Europe.
However, whilst there is compelling evidence for the hunting of wooly mammoths and large ungulates by Middle Paleolithic populations, there is also a growing body of evidence to suggest Neanderthal diet may have been broader than previously argued. First, the isotopic evidence used to construct the trophic position of Neanderthal populations has been questioned. This is because nitrogen isotope data only provides evidence for protein consumption. This leads to a significant undercalculation of plant food use in diets, because such resources usually contribute to carbohydrate and fat, rather than protein, intake. Even when specifically investigating the percentage contribution of animal and plant foods to protein intake, the analysis of nitrogen isotopes from bulk collagen samples has been shown to underestimate the significance of plant foods (Wißing et al. 2016). Where analysis has instead been completed on individual amino acids, estimations of plant food contributions to protein intake have been significant even in cold environments (e.g., >20% at Buran Kaya III, south Crimea: Drucker et al. 2017; up to 20%, at Spy Cave, Belgium: Naito et al. 2016). Further, this isotopic data is preferentially available at archaeological sites from colder climates, where plants likely played a smaller role in Neanderthal diets, as warmer climates are less conducive to the survival of ancient collagen in human bones (Power and L‘Engle Williams 2018).
Second, there is a growing body of direct evidence for a significant contribution of plant foods and fungi to Neanderthal diets (see Table 1). Across Eurasia, microfossils and eukaryotic aDNA preserved in dental calculus have attested to the likely consumption of a range of plant foods and fungi by Neanderthals, including fruits, nuts, wild grass seeds, legumes, USOs, and mushrooms (Henry et al. 2011, 2014; Weyrich et al. 2017; Power et al. 2018; Salazar-García et al. 2021). Whilst this data does not attest to the proportion of plant foods eaten by Neanderthal populations, direct comparison to modern human dental calculus from Middle and Upper Paleolithic Europe has shown no obvious difference either in the range of plant taxa consumed or the evidence for cooking practices (Henry et al. 2014). Further to this, phytolith analysis of sediment samples at Amud Cave, Israel, provides clear and repetitive evidence for the collection and likely consumption of grass seeds by its Neanderthal population, 70-55 kya (Madella et al. 2002), and recent macrobotanical evidence from Figueira Brava Cave, Portugal, provides evidence for continued harvesting of pine nuts, requiring the climbing of mature pine trees, 10686 kya (Zilhão et al. 2020). The latter is one of several Neanderthal sites to provide macrobotanical evidence for the exploitation of fatty tree nuts and fruits (see Table 1; Barton et al. 1999; Lev et al. 2005). It is, therefore, likely that plant foods made a more significant contribution to Neanderthal diets than previously thought, especially in the more southern range of their distribution. Third, there is also evidence for the exploitation of significant quantities of small vertebrates, avifauna, fish, and marine mammals and shellfish by some Neanderthal populations in the southerly latitudes of Europe (Stringer et al. 2008; Blasco and Fernández Peris 2012; Will et al. 2019; Zilhão et al. 2020).
This evidence suggests that the large-game hunting focus of Neanderthals in high-latitude Europe was likely supported by the consumption of carbohydrates and fats from plant foods, especially in late winter when animal prey had lower body fats (Jones 2009; Hardy 2010). Further, much like early modern human populations, Neanderthals likely exploited a range of resources dependent on their local environment, including hard-to-process plant foods, such as grass seeds, fish and animal prey requiring a range of capture technologies. This does not, however, negate a general trend visible in the Upper Paleolithic, which saw continued increases in the contribution of small game to modern human diets (Stiner 2001), and a range of technological changes, including the increased use of grinding technologies. Power and L’Engle Williams (2018) reviewed the archaeological evidence for increases in grinding stones in the Upper Paleolithic, and found an increase in this technology beyond that hypothesized for increasing population size in this period. They argued this archaeologically-more-robust evidence for plantprocessing indicated increasing processing practices in the Upper Paleolithic, and especially following the Last Glacial Maximum. This may be linked to an increased gendered division of labor in early modern human populations. Alongside other factors, such as the local availability of plant foods with changing climate, increasingly distinct economic roles may have allowed for the individual technological specialization required to produce a broader group diet. However, this appears to be a more gradual and spatially diverse pattern than originally proposed by Kuhn and Stiner (Kuhn and Stiner 2006; Stiner and Kuhn 2009), and a continuation of foraging practices already displayed by Neanderthals. It is, therefore, hard on present evidence to conclude as to whether an increased gendered division of labor and specialization in plants and other foods was responsible for the out-competition of Neanderthals in Eurasia, or if its increased archaeological visibility was an effect of increasing modern human population size following Neanderthal extinction (Martínez 2006).
Diet and peopling of the Wallacean Archipelago and sahul
The Wallacean archipelago and Sahul provide a valuable case study for further disentangling the early diets, plant food use and adaptive capacities of modern humans and other hominin species. The earliest evidence for hominin dispersal into this region comes from stone tools on the island of Flores 1 mya (Brumm et al. 2010; see also van den Bergh et al. 2016a), the Philippines 709 kya (Ingicco et al. 2018) and Sulawesi 200 kya (van den Bergh et al. 2016b). These artifacts are likely the result of H. erectus making limited water-crossings onto these island environments, alongside several proboscid and rodent species (Dennell et al. 2014; Gaffney 2020). These appear to be outlier events, genetic restrictions from Asian mainland populations eventuating in the allopatric speciation of dwarfed hominins, including H. floresiensis and H. luzonensis, and there is no evidence for successful water-crossings into the smaller and more faunally depauperate islands of the Wallacean Archipelago. Recent genetic evidence for admixture with early Australian and New Guinean populations, also suggests Denisovans may have been present in the Wallacean Archipelago (Carlhoff et al. 2021; Teixeira et al. 2021). However, there is no definitive fossil evidence for this species outside the Altai Mountains. Where evidence for diet is available for H. floresiensis, they appear to have been restricted to terrestrial resources, likely including stegodons and rats (van den Bergh et al. 2009; Brumm et al. 2016; Sutikna et al. 2016; O’Connor et al. 2017). This is despite some evidence from Java for the exploitation of shellfish by H. erectus (Choi and Driwantoro 2007; Joordens et al. 2009). Interestingly, at Liang Bua, Flores, there is no micromorphological evidence for the presence of hearths within the archaeological layers associated with H. floresiensis, suggesting cooking may not have been a part of their behavioral toolkit (Morley et al. 2017).
Early evidence for modern humans in this region has been argued to be markedly different, with early populations relying on marine resources to people smaller Wallacean islands, and mainland Sahul (O’Connor et. al. 2017; Allen and O’Connell 2020; Shipton et al. 2021). Early evidence for H. sapiens diet at Laili Cave, 44.6 kya, Asitau Kuru, 44 kya and Lene Hara Cave, 42 kya, on Timor, and Makpan, 40 kya, on Alor Island attests to the exploitation of marine shellfish, fish, including fast-moving pelagic species (O’Connor et al. 2011; cf. Anderson 2013), sea turtle, crab and urchin (Hawkins et al. 2017; O’Connor et al. 2017; Shipton et al. 2019; Kealy et al. 2020). This is supported by isotopic evidence from a H. sapiens tooth recovered from earliest occupation at Asitau Kuru, which indicates a reliance on marine resources (Roberts et al. 2020). This evidence has been used to characterize early modern human movement through Wallacea as rapid and underpinned by the pull of high-ranked, yet easily over-predated, coastal resources (O’Connell and Allen 2012). Populations with narrow diets in the early stages of expansion, chose to relocate to new coastal habitats rather than invest in more labor-intensive resource acquisition. However, evidence from other early sites in Wallacea suggest early modern humans exploited inland environments at similar or, even, earlier time depths. The earliest figurative art in this region, dating to 45.5 kya at Leang Tedongnge and 43.9 kya at Leang Bulu’ Sipong 4, Sulawesi, depicts wild pigs and dwarf bovids hunted by therianthrops (Aubert et al. 2019; Brumm et al. 2021). Zooarchaeological evidence from inland archaeological sites in Flores (Liang Bua, 4746 kya), Sulawesi (Leang Sakapao 1 and Leang Burung 2, ~36 kya), and Timor (Matja Kuru 2, 36.3-35 kya) attributed to H. sapiens suggest a reliance on terrestrial resources (Bulbeck et al. 2004; O’Connor et al. Aplin 2014; Sutikna et al. 2016; Brumm et al. 2018). Further, early occupation at Niah Cave, Borneo, 46-34 kya, then part of mainland Asia, demonstrates adaptation to tropical forest, predicated on the exploitation of a wide range of resources, including pigs, small primates, and plant resources (Barton 2005; Barker et al. 2007; Barton and Paz 2007). This included plant foods that required processing, including leaching for toxicity, such as wild yam (cf. Dioscorea hispida) and kepayang (Pangium edule) nuts. This is in keeping with other archaeological evidence from across the southern dispersal arc, which demonstrates early adaptations to a range of non-coastal environments, including rainforest (Roberts et al. 2017; Wedage et al. 2019a), high-altitude (Fairbairn et al. 2006; Summerhayes et al. 2010) and arid environments (Blinkhorn et al. 2017; Groucutt et al. 2018, 2021). Rather than providing evidence for the pull factors of high-calorie, easy-to-capture resources available to foragers in Wallacea, O’Connor et al. (2017) argue that evidence for the use of a range of shellfish, fish and other marine resources by early modern humans in the Wallacean Archipelago demonstrates their adaptive capacity and dietary flexibility. In comparison to Sulawesi, Luzon and Flores, the smaller islands of Wallacea have few large- and medium-bodied terrestrial fauna, and their settlement in the Pleistocene and Holocene was almost entirely reliant on marine fauna until the introduction of domesticated plants and animals (Samper Carro et al. 2016; Hawkins et al. 2017). Whilst some of these resources are easily harvested (e.g., tridacnids; O’Connell and Allen 2012), many also require specialized technologies, such as linefishing (evidenced by 23-16 kya; O’Connor et al. 2011). Alongside the technological requirements of watercraft, and the cognitive capacities for planning and forethought requisite for longer sea-voyages made by early modern humans (Leppard 2015; Shipton et al. 2021), the ability to exploit a broad range of marine resources is argued to have both underpinned successful Homo sapiens dispersal and limited earlier hominin movements through Wallacea (O’Connor et al. 2017; Gaffney 2020).
The importance of plant foods for early movement in Wallacea and Sahul
Whether a pull factor or a demonstration of adaptive flexibility, a heavy reliance on lean, marine proteins, such as shellfish and fish, to people the Wallacean Archipelago must necessarily have been supported by access to fats and carbohydrates from other sources (Noli and Avery 1988). With a lack of large-and medium-bodied fauna on smaller islands, this means early modern humans would have had to, at least in part, rely on plant resources for these nutrients. Due to poor preservation and a lack of systematic archaeobotanical recovery, there is, however, no direct evidence for Pleistocene plant food use in the Wallacean Archipelago by H. sapiens or earlier hominin populations. However, early archaeobotanical evidence from northern Sahul indicates that early human populations used and processed a wide range of plant foods shared across the Wallacean Archipelago.
The earliest archaeobotanical evidence from Sahul is recovered from Phase 2 at Madjedbebe, northern Australia, dating from 65-52.7 kya (Clarkson et al. 2017; Florin et al. 2020). This phase, representing the earliest known archaeological evidence for humans in Sahul, includes evidence for complex technologies, such as grinding stones and ground-edge axes, the material evidence of symbolic behavior, including the production of a compound, reflective (micaceous) pigment, and evidence for a broad plant diet. The latter includes macrobotanical, and residue and usewear evidence for the use of a range of plant foods and processing techniques, including cooking, pounding and grinding (Florin et al. 2020). There is no evidence for the animal component of the diet in this phase. However, as most Australian fauna, and wild animals globally, are low in fat (Naughton et al. 1986), it can be inferred that it consisted of mostly lean proteins. It is, therefore, unsurprising that considerable labor was expended on the extraction of polydrupe pandanus (Pandanus spiralis) kernels, which are rich in fat (44-50%; Low 1991), and that a range of plant carbohydrates, including USOs and palm pith, requiring roasting and pounding (RussellSmith et al. 1997), and, likely, seeds were exploited.
Many of the genera known to be exploited at Madjedbebe (Buchanania, Canarium, Livistona, Pandanus and Terminalia) are shared across the Wallacean Archipelago and, in many cases, are also found in tropical and sub-tropical vegetation across the southern dispersal arc (Golson 1971; Crisp et al. 2010; van Welzen et al. 2011). The same is true of plant foods used at other early archaeological sites in northern Sahul, including Terminalia sp. and black plum (Vitex cf. glabrata) fruits and nuts, from Carpenter’s Gap 1, northern Australia, 51-38.8 kya (McConnell and O’Connor 1997; Dilkes-Hall et al. 2019), and monodrupe pandanus (Pandanus cf. taip) and yam (cf. Dioscorea sp.) tubers from the Ivane Valley, highland New Guinea, 49-36 kya (Fairbairn et al. 2006; Summerhayes et al. 2010). The latter evidence even suggests the transportation of low-altitude yams into the New Guinea highlands to support the early use of this region. It is, therefore, likely that early human populations drew upon their culturally transmitted knowledge of the ecology and processing requirements of tropical plant foods, as well as cognitive capacity to apply this knowledge flexibly to novel species and environments, to move across the Wallacean Archipelago and into Sahul. Alongside dietary flexibility and marine-specialized technologies, the ability to extract nutrients from a broad range of plant foods, would have allowed early humans to adapt to a range of environments, including marginal zones, such as faunally depauperate islands and high-latitudes.
H. floresiensis and H. luzonensis species likely also used a range of plant foods. However, there is little evidence in Wallacea to suggest they routinely cooked, or otherwise used complex technologies to process plant foods, and some evidence to suggest they did not (Morley et al. 2017). This may be an artifact of preservation as direct evidence for plant food use is sparse. However, it may also be that these activities are part of a gradual development of dietary specialization and flexibility epitomized by H. sapiens, but also evidenced in Neanderthals (and likely Denisovans), that allowed for adaptations to a range of environments globally.
Labor specialization in Sahul
Alongside evidence for a broad plant diet at Madjedbebe, there is also evidence for specialized technology, including grinding stones and ground-edge axes, from the earliest phase of occupation (Clarkson et al. 2017). The latter of these technologies, ground-edge axes, is a multi-purpose tool requiring both a high-level of knapping skill, and a large investment of labor to produce and grind (Hiscock et al. 2016; Shipton et al. 2020; Ford and Hiscock 2021). As such, this technology suggests a specialized division of labor for its production. The presence of these technologies and a broad plant diet may, therefore, point to a specialized and gendered division of labor like that argued for the Upper Paleolithic in Eurasia (Kuhn and Stiner 2006; Stiner and Kuhn 2009), albeit without its extensive osseous record. This would suggest early modern humans peopling Australia by 65 kya had specialized economic roles, allowing them to adeptly exploit a range of resources, including hard-to-process plant foods. Neither grinding stones nor ground-edge axes are, however, part of the archaeological record in Pleistocene Wallacea (Shipton et al. 2020) and, in the case of ground-edge axes, they are also limited to northern Sahul in the Pleistocene (Ford and Hiscock 2021). As such, these technologies are at present understood regionally to be innovations occurring with the peopling of northern Australia. Whilst this may suggest that intensive plant processing and specialized division of labour also only occurred with the peopling of Sahul, given the extent of plant-processing evidenced at Niah Cave without grinding stone technology, it is more likely that these more archaeologically visible technologies cannot be relied upon to denote such behavior. Many technologies associated with plant processing and other specialized labor are ethnographically known to have been made with less durable materials, and even the grinding of grass seeds need not have always required specialized stone technologies (e.g., Mercader 2009). Absence of evidence for specialized labor roles and dietary breadth cannot be equated with evidence for absence, and the role of specialized archaeobotanical recovery methods and analysis must not be underestimated if we are to better understand such practices in Wallacea and Sahul, and indeed, globally.
Conclusion
Evidence for the use of plant foods by early modern humans and our closest ancestors from Africa, Eurasia, Wallacea and Sahul, suggests plant foods and processing technologies, such as cooking, grinding and leaching, were important to people’s dietary flexibility and adaptive capacity. The necessity for fats and carbohydrates in the diets of hominins means that, except in extreme and mostly recent circumstances (e.g., arctic living and pastoralism), plant foods would have been required in considerable amounts. The ability to process nutrients available within plants effectively allowed for increased dietary breadth, and likely, alongside the ability to better exploit a large range of animal foods, underpinned the ability of our species to move into new and marginal environments globally. This appears especially true of the early movement into Sahul, where a broad plant diet and associated processing technology is evidenced with first peopling. However, like many traits considered to be behaviorally modern it is increasingly apparent that plant processing was not unique to our species. Indeed, with increased direct evidence for Neanderthal plant food use, there is a growing awareness of similarities in the dietary niche of modern and archaic humans in Eurasia. Importantly, if we are to further elucidate the role of plant foods in hominin evolution and dispersals, it is critical that systematic archaeobotanical recovery and analysis of macro- and micro-fossils becomes a universal practice in Paleolithic archaeology globally.
Acknowledgements
This paper was in part drawn from unpublished portions of my PhD dissertation, ‘Archaeobotanical investigations into 65,000 years of plant food and landscape use at Madjedbebe, Mirarr Country, northern Australia’, including discussions I had with Graeme Barker and Dorian Fuller during its defense. I thank my examiners, my supervisors, Chris Clarkson and Andrew Fairbairn, and Alison Crowther for many thoughtful conversations pertaining to this research. I also thank Erin Mein for help producing Figure 1, and Kasih Norman, Dylan Gaffney, and an anonymous reviewer for their perceptive comments on the manuscript. All mistakes are, however, my own. My PhD research was possible thanks to the support of the Mirarr people, and I thank the Senior Traditional Owners, Yvonne Margarula and May Nango, and the Gundjeihmi Aboriginal Corporation for permission to carry out research at Madjedbebe. I also thank the Australian Research Council (ARC; Research Training Program Scholarship), the Australian Institute of Nuclear Science and Engineering (Postgraduate Research Award 11877), the Wenner Gren Foundation (Dissertation Fieldwork Grant 9260), the Dan David Foundation (Scholarship) and the ARC Centre of Excellence for Australian Biodiversity and Heritage (Irinjili Research Training Program Internship for Women) for funding my PhD research. Finally, I would like to thank the University of Tübingen committee for awarding me the 2021 Tübingen Research Prize for Early Prehistory and Quaternary Ecology.
References
Aiello, L. C. and Wheeler, P. 1995: The Expensive-Tissue Hyothesis. The Brain and the Digestive System in Human and Primate Evolution. Current Anthropology 36, 199–221.
Aigner, J. S. 1969: The Archaeology of Pleistocene China. Madison: University of Wisconsin.
Allen, J. and O’Connell, J. F. 2020: A Different Paradigm for the Initial Colonisation of Sahul. Archaeology in Oceania 55,1–14.
Allué, E., Cáceres, I., Expósito, I., Canals, A., Rodríguez, A., Rosell, J., Bermúdez de Castro, J. M., and Carbonell, E. 2015: Celtis Remains from the Lower Pleistocene of Gran Dolina, Atapuerca (Burgos, Spain). Journal of Archaeological Science 53, 570–577.
Anderson, A. 2013: The Antiquity of Sustained Offshore Fishing. Antiquity 87, 879–855.
Arbuckle, B. S. and Hammer, E. L. 2018: The Rise of Pastoralism in the Ancient Near East. Journal of Archaeological Research 27, 391–449.
Atkinson, F. S., Hancock, D., Petocz, P., and Brand-Miller, J. C. 2018: The Physiologic and Phenotypic Significance of Variation in Human Amylase Gene Copy Number. American Journal of Clinical Nutrition 108, 737–748.
Aubert, M., Lebe, R., Oktaviana, A. A., Tang, M., Burhan, B., Hamrullah, Jusdi, A., Abdullah, Hakim, B., Zhao, J.-x., Geria, I. M., Sulistyarto, P. H., Sardi, R., and Brumm, A. 2019: Earliest Hunting Scene in Prehistoric Art. Nature 576, 442–445.
Barker, G., Barton, H., Bird, M., Daly, P., Datan, I., Dykes, A., Farr, L., Gilbertson, D., Harrisson, B., Hunt, C., Higham, T., Kealhofer, L., Krigbaum, J., Lewis, H., McLaren, S., Paz, V., Pike, A., Piper, P., Pyatt, B., Rabett, R., Reynolds, T., Rose, J., Rushworth, G., Stephens, M., Stringer, C., Thompson, J., and Turney, C. 2007: The ‘Human Revolution’ in Lowland Tropical Southeast Asia: The Antiquity and Behavior of Anatomically Modern Humans at Niah Cave (Sarawak, Borneo). Journal of Human Evolution 52, 243–261.
Barton, H. 2005: The Case for Rainforest Foragers: The Starch Record at Niah Cave, Sarawak. Asian Perspectives 44, 56–72.
Barton, H. and Paz, V. 2007: Subterranean Diets in the Tropical Rainforests of Sarawak, Malaysia. In: T. P. Denham, J. Iriarte, and L. Vrydaghs (eds.), Rethinking Agriculture. Archaeological and Ethnoarchaeological Perspectives. Walnut Creek: Left Coast Press, 50–77.
Barton, R. N. E., Currant, A. P., Fernandez-Jalvo, Y., Finlayson, J. C., Goldberg, P., Macphail, R., Pettitt, P. B., and Stringer, C. B. 1999: Gibraltar Neanderthals and Results of Recent Excavations in Gorham’s, Vanguard, and Ibex Caves. Antiquity 73, 13–23.
Bellomo, R. V. 1994: Methods of Determining Early Hominid Behavioral Activities Associated with the Controlled Use of Fire at FxJj 20 Main, Koobi Fora, Kenya. Journal of Human Evolution 27, 173–195.
Ben-Dor, M. and Barkai, R. 2021: Prey Size Decline as a Unifying Ecological Selecting Agent in Pleistocene Human Evolution. Quaternary 4, 7. https://doi.org/10.3390/quat4010007.
Ben-Dor, M., Gopher, A., Hershkovitz, I., and Barkai, R. 2011: Man the Fat Hunter: The Demise of Homo erectus and the Emergence of a New Hominin Lineage in the Middle Pleistocene (ca. 400 kyr) Levant. PLoS ONE 6(12): e28689.
Ben-Dor, M., Sirtoli, R., and Barkai, R. 2021: The Evolution of the Human Trophic Level During the Pleistocene. Yearbook of Physical Anthropology 175 (Suppl. 72), 27–56.
Berna, F., Goldberg, P., Horwitz, L. K., Brink, J., Holt, S., Bamford, M., and Chazan, M. 2012: Microstratigraphic Evidence of in situ Fire in the Acheulean Strata of Wonderwerk Cave, Northern Cape province, South Africa. Proceedings of the National Acadamy of Sciences of the U.S.A 109(20), E1215–E1220.
Billsborough, S. and N. Mann. 2006 A Review of Issues of Dietary Protein Intake in Humans. International Journal of Sport Nutrition and Exercise Metabolism 16(2),129–152.
Binford, L. R. and Ho, C. K. 1985: Taphonomy at a Distance: Zhoukoudian, “The Cave Home of Beijing Man”? Current Anthropology 26, 413-442.
Bittmann, F. 1992: The Kärlich interglacial, Middle Rhine region, Germany: vegetation history and stratigraphic position. Vegetation History and Archaeobotany 1, 243–258.
Blasco, R. and Fernández Peris, J. 2012: A Uniquely Broad Spectrum Diet During the Middle Pleistocene at Bolomor Cave (Valencia, Spain). Quaternary International 252, 16–31.
Blinkhorn, J., Achyuthan, H., Ditchfield, P., and Petraglia, M. 2017: Palaeoenvironmental Dynamics and Palaeolithic Occupation at Katoati, Thar Desert, India. Quaternary Research 87, 298–313.
Bocherens, H. 2009: Neanderthal Dietary Habits: Review of the Isotopic Evidence. In: J.-J. Hublin and M. P. Richards (eds.),The Evolution of Hominin Diets. Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht: Springer, 241–250.
Bocherens, H., Drucker, D. G., Billiou,D., Patou-Mathis, M., and Vandermeersch, B. 2005: Isotopic Evidence for Diet and Subsistence Pattern of the Saint-Cesaire I Neanderthal: Review and Use of a Multi-Source Mixing Model. Journal of Human Evolution 49, 71–87.
Brain, C. K. and Sillen, A. 1988: Evidence from the Swartkrans Cave for the Earliest Use of Fire. Nature 336, 464–466.
Bramble, D. M. and Lieberman, D. E. 2004: Endurance Running and the Evolution of Homo. Nature 432, 345–352.
Brown, P., Sutikna, T., Morwood, M. J., Soejono, R. P., Jatmiko, Saptomo, E. W., and Due, R. A. 2004: A New Small-Bodied Hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061.
Brumm, A., Jensen, G. M., van den Bergh, G. D., Morwood, M. J., Kurniawan, I., Aziz, F., and Storey, M. 2010: Hominins on Flores, Indonesia, by One Million Years Ago. Nature 464, 748–752.
Brumm, A., van den Bergh, G. D., Storey, M., Kurniawan, I., Alloway, B. V., Setiawan, R., Setiyabudi, E., Grün, R., Moore, M. W., Yurnaldi, D., Puspaningrum, M. R., Wibowo, U. P., Insani, H., Sutisna, I., Westgate, J. A., Pearce, N. J. G., Duval, M., Meijer, H. J. M., Aziz, F., Sutikna, T., van der Kaars, S., Flude, S., and Morwood, M. J. 2016: Age and Context of the Oldest Known Hominin Fossils from Flores. Nature 534, 249–253.
Brumm, A., Hakim, B., Ramli, M., Aubert, M., van den Bergh, G. D., Li, B., Burhan, B., Saiful, A. M., Siagian, L., Sardi, R., Jusdi, A., Abdullah, Mubarak, A. P., Moore, M. W., Roberts, R. G., Zhao, J.-x., McGahan, D., Jones, B. G., Perston, Y., Szabó, K., Mahmud, M. I., Westaway, K., Jatmiko, Saptomo, E. W., van der Kaars, S., Grün, R., Wood, R., Dodson, J., and Morwood, M. J. 2018: A Reassessment of the Early Archaeological Record at Leang Burung 2, a Late Pleistocene Rock-Shelter Site on the Indonesian Island of Sulawesi. PLoS ONE 13(4): e0193025.
Brumm, A., Oktaviana, A. A., Burhan, B., Hakim, B., Lebe, R., Zhao, J.-x., Sulistyarto, P. H., Ririmasse, M., Adhityatama, S., Sumantri, I., and Aubert, M. 2021: Oldest Cave Art Found in Sulawesi. Science Advances 7: eabd4648.
Bulbeck, D., Sumantri, I., and Hiscock, P. 2004: Leang Sakapao 1, A Second Dated Pleistocene Site from South Sulawesi, Indonesia. In: S. G. Keates and J. M. Pasveer (eds.), Quaternary Research in Indonesia. Leiden: A. A. Balkema Publishers, 111–128.
Bunn, H. T. 1981: Archaeological Evidence for Meat-Eating by Plio-Pleistocene Hominids from Koobi Fora and Olduvai Gorge. Nature 291, 574–577.
Bunn, H. T. 2007: Meat Made Us Human. In: P. S. Ungar (ed.), Evolution of the Human Diet. The Known, the Unknown and the Unknowable. Oxford: Oxford University Press, 191–211.
Burke, A. 2000: The View from Starosele: Faunal Exploitation at a Middle Palaeolithic Site in Western Crimea. International Journal of Osteoarchaeology 10, 325–335.
Carlhoff, S., Duli, A., Nägele, K., Nur, M., Skov, L., Sumantri, I., Oktaviana, A. A., Hakim, B., Burhan, B., Syahdar, F. A., McGahan, D. P., Bulbeck, D., Perston, Y. L., Newman, K., Saiful, A. M., Ririmasse, M., Chia, S., Hasanuddin, Pulubuhu, D.A. T., Suryatman, Supriadi, Jeong, C., Peter, B. M., Prüfer, K., Powell, A., Krause, J., Posth, C., and Brumm, A. 2021: Genome of a Middle Holocene Hunter-Gatherer from Wallacea. Nature 596, 543–547.
Carmody, R. N. and Wrangham. R. W. 2009: The Energetic Significance of Cooking. Journal of Human Evolution 57, 379–391.
Chaney, R. W. 1935: The Occurrence of Edocarps of Celtis barbouri at Choukoutien. Bulletin of the Geological Society of China 14, 99–118.
Choi, K. and Driwantoro, D. 2007: Shell Tool Use by Early Members of Homo erectus in Sangiran, Central Java, Indonesia: Cut Mark Evidence. Journal of Archaeological Science 34, 48–58.
Clarkson, C., Jacobs, Z., Marwick, B., Fullagar, R., Wallis, L., Smith, M., Roberts, R. G., Hayes, E., Lowe, K., Carah, X., Florin, S. A., McNeil, J., Cox, D., Arnold, L. J., Hua, Q., Huntley, J., Brand, H. E. A., Manne, T., Fairbairn, A., Shulmeister, J., Lyle, L., Salinas, M., Page, M., Connell, K., Park, G., Norman, K., Murphy, T., and Pardoe, C. 2017: Human Occupation of Northern Australia by 65,000 Years Ago. Nature 547, 306–310.
Cordain, L., Miller, J. B., Eaton, S. B., Mann, N., Holt, S. H., and Speth, J. D. 2000: Plant-Animal Subsistence Ratios and Macronutrient Energy Estimations in Worldwide Hunter-Gatherer Diets. American Journal of Clinical Nutrition 71, 682–692.
Crisp, M. D., Isagi, Y., Kato, Y., Cook, L. G., and Bowman, D. M. J. S. 2010: Livistona Palms in Australia: Ancient Relics or Opportunistic Immigrants? Molecular Phylogenetics and Evolution 54, 512–523.
de Lumley, H., de Lumley, M. A., Miskovsky, J. C., and Renault-Miskovsky, J. 1976: La grotte du Lazaret. Livret-Guide B1 de l’UISPP: Sites paléolithiques de la région de Nice et grottes de Grimaldi, IX Congress, 53–74.
Dennell, R. W., Louys, J., O’Regan, H. J., and Wilkinson, D. M. 2014: The Origins and Persistence of Homo floresiensis on Flores: Biogeographical and Ecological Perspectives. Quaternary Science Reviews 96, 98–107.
Détroit, F., Mijares, A. S., Corny, J., Daver, G., Zanolli, C., Dizon, E.,Robles, E., Grün, R., and Piper, P. J. 2019: A New Species of Homo from the Late Pleistocene of the Philippines. Nature 568, 181–186.
Dilkes-Hall, I. E., O’Connor, S., and Balme, J. 2019: People-Plant Interaction and Economic Botany over 47,000 Years of Occupation at Carpenter’s Gap 1, South Central Kimberley. Australian Archaeology 85, 30–47.
Domínguez-Rodrigo, M. and Pickering, T. R. 2003: Early Hominid Hunting and Scavenging: A Zooarcheological Review. Evolutionary Anthropology 12, 275–282.
Domínguez-Rodrigo, M., Pickering, T. R., Semaw, S., and Rogers, M. J. 2005: Cutmarked Bones from Pliocene
Archaeological Sites at Gona, Afar, Ethiopia: Implications for the Function of the World’s Oldest Stone Tools. Journal of Human Evolution 48, 109–121.
Doran, D. M., McNeilage, A., Greer, D., Bocian, C., Mehlman, P., and Shah, N. 2002: Western Lowland Gorilla Diet and Resource Availability: New Evidence, Cross-Site Comparisons, and Reflections on Indirect Sampling Methods. American Journal of Primatology 58(3), 91–116.
Drucker, D. G., Naito, Y. I., Péan, S., Prat, S., Crépin, L., Chikaraishi, Y., Ohkouchi, N., Puaud, S., Lázničková-Galetová, M., Patou-Mathis, M., Yanevich, A., and Bocherens, H. 2017: Isotopic Analyses Suggest Mammoth and Plant in the Diet of the Oldest Anatomically Modern Humans from Far Southeast Europe. Scientific Reports 7: 6833.
Fairbairn, A. S., Hope, G. S., and Summerhayes, G. R. 2006: Pleistocene Occupation of New Guinea’s Highland and Subalpine Environments. World Archaeology 38, 371–386.
Fellows Yates, J. A., Velsko, I. M., Aron, F., Posth, C., Hofman, C. A., Austin, R. M., Parker, C. E., Mann, A. E., Nägele, K., Weedman Arthur, K., Arthur, J. W., Bauer, C. C., Crevecoeur, I., Cupillard, C., Curtis, M. C., Dalén, L., DíazZorita Bonilla, M., Díez Fernández-Lomana, J. C., Drucker, D. G., Escribano Escrivá, E., Francken, M., Gibbon, V. E., González Morales, M. R., Grande Mateu, A., Harvati, K., Henry, A. G., Humphrey, L., Menéndez, M., Mihailović, D., Peresani, M., Rodríguez Moroder, S., Roksandic, M., Rougier, H., Sázelová, S., Stock, J. T., Straus, L. G., Svoboda, J., Teßmann, B., Walker, M. J., Power, R. C., Lewis, C. M., Sankaranarayanan, K., Guschanski, K., Wrangham, R. W., Dewhirst, F. E., Salazar-García, D. C., Krause, J., Herbig, A., and Warinner, C. 2021: The Evolution and Changing Ecology of the African Hominid Oral Microbiome. Proceedings of the National Academy of Sciences of the U.S.A. 118 No 20: e2021655118.
Flegontov, P., Altınışık, N. E., Changmai, P., Rohland, N., Mallick, S., Adamski, N., Bolnick, D. A., Broomandkhoshbacht, N., Candilio, F., Culleton, B. J., Flegontova, O., Friesen, T. M., Jeong, C., Harper, T. K., Keating, D., Kennett, D. J., Kim, A. M., Lamnidis, T. C., Lawson, A. M., Olalde, I., Oppenheimer, J., Potter, B. A., Raff, J., Sattler, R. A., Skoglund, P., Stewardson, K., Vajda, E. J., Vasilyev, S., Veselovskaya, E., Hayes, M. G., O’Rourke, D. H., Krause, J., Pinhasi, R., Reich, D., and Schiffels, S. 2019: Palaeo-Eskimo Genetic Ancestry and the Peopling of Chukotka and North America. Nature 570, 236–240.
Florin, S. A., Fairbairn, A. S., Nango, M., Djandjomerr, D., Marwick, B., Fullagar, R., Smith, M., Wallis, L. A., and Clarkson, C. 2020: The First Australian Plant Foods at Madjedbebe, 65,000–53,000 Years Ago. Nature Communications 11: 924.
Ford, A. and Hiscock, P. 2021: Axe Quarrying, Production, and Exchange in Australia and New Guinea. In: I. J. McNiven and B. David (eds.), The Oxford Handbook of the Archaeology of Indigenous Australia and New Guinea. Oxford: Oxford University Press. DOI: 10.1093/oxfordhb/9780190095611.013.22.
Gabunia, L., Vekua, A., and Lordkipanidze, D. 2000: The Environmental Contexts of Early Human Occupation of Georgia (Transcaucasia). Journal of Human Evolution 38, 785–802.
Gaffney, D. 2020: Pleistocene Water Crossings and Adaptive Flexibility Within the Homo Genus. Journal of Archaeological Research 29, 255–326.
Gaudzinski, S. 1995: Wallertheim Revisited: A Re-analysis of the Fauna from the Middle Palaeolithic Site of Wallertheim (Rheinhessen/Germany). Journal of Archaeological Science 22, 51–66.
Golson, J. 1971: Australian Aboriginal Plant Foods: Some Ecological and Cultural-Historical Implications. In: D. J. Mulvaney and J. Golson, Aboriginal Man and Environment in Australia. Canberra: Australian National University Press, 196–238.
Goren-Inbar, N., Sharon, G., Melamed, Y., and Kislev, M. 2002: Nuts, Nut Cracking, and Pitted Stones at Gesher Benot Ya‘aqov, Israel. Proceedings of the National Academy of Sciences of the U.S.A. 99, 2455–2460.
Griggo, C. 2004: Mousterian Fauna from Dederiyeh Cave and Comparisons with Fauna from Umm El Tlel and Douara Cave. Paléorient 30/1, 149–162.
Groucutt, H. S., Grün, R., Zalmout, I. A. S., Drake, N. A., Armitage, S. J., Candy, I., Clark-Wilson, R., Louys, J., Breeze, P. S., Duval, M., Buck, L. T., Kivell, T. L., Pomeroy, E., Stephens, N. B., Stock, J. T., Stewart, M., Price, G. J., Kinsley, L., Sung, W. W., Alsharekh, A., Al-Omari, A., Zahir, M., Memesh, A. M., Abdulshakoor, A. J., Al-Masari, A. M., Bahameem, A. A., Al Murayyi, K. M. S., Zahrani, B., Scerri, E. L. M., and Petraglia, M. D. 2018: Homo sapiens in Arabia by 85,000 years ago. Nature Ecology & Evolution 2, 800–809.
Groucutt, H. S., White, T. S., Scerri, E. M. L., Andrieux, E., Clark-Wilson, R., Breeze, P. S., Armitage, S. J., Stewart, M., Drake, N., Louys, J., Price, G. J., Duval, M., Parton, A., Candy, I., Carleton, W. C., Shipton, C., Jennings, R. P., Zahir, M., Blinkhorn, J., Blockley, S., Al-Omari, A., Alsharekh, A. M., and Petraglia, M. D. 2021: Multiple Hominin Dispersals into Southwest Asia over the Past 400,000 Years. Nature 597, 376–380.
Hardy, B. L. 2010: Climatic Variability and Plant Food Distribution in Pleistocene Europe: Implicationsfor Neanderthal Diet and Subsistence. Quaternary Science Reviews 29, 662–679.
Hardy, K., Buckley, S., Collins, M. J., Estalrrich, A., Brothwell, D., Copeland, L., García-Tabernero, A., García-Vargas, S., de la Rasilla, M., Lalueza-Fox, C., Huguet, R., Bastir, M., Santamaría, D., Madella, M., Wilson, J., Fernández Cortés, Á., and Rosas, A. 2012: Neanderthal Medics? Evidence for Food, Cooking, and Medicinal Plants Entrapped in Dental Calculus. Naturwissenschaften 99, 617–626.
Hardy, K., Brand-Miller, J., Brown, K. D., Thomas, M. G., and Copeland, L. 2015: The Importance of Dietary Carbohydrate in Human Evolution. The Quarterly Review of Biology 90, 251–268.
Hardy, K., Radini, A., Buckley, S., Blasco, R., Copeland, L., Burjachs, F., Girbal, J., Yll, R., Carbonell, E., and Bermúdez de Castro, J. M. 2017: Diet and Environment 1.2 Million Years Ago Revealed Through Analysis of Dental Calculus from Europe’s Oldest Hominin at Sima del Elefante, Spain. The Science of Nature/Naturwissenschaften 104: 2.
Hawkes, K., O’Connell, J. F., and Blurton Jones, N. G. 1991: Hunting Income Patterns among the Hadza: Big Game, Common Goods, Foraging Goals and the Evolution of the Human Diet. Philosophical Transactions of the Royal Society B 334, 243–251.
Hawkins, S., O’Connor, S., Maloney, T. R., Litster, M., Kealy, S., Fenner, J. N., Aplin, K., Boulanger, C., Brockwell, S., Willan, R., Piotto, E., and Louys, J. 2017: Oldest Human Occupation of Wallacea at Laili Cave, Timor-Leste, Shows Broad-Spectrum Foraging Responses to Late Pleistocene Environments. Quaternary Science Reviews 171, 58–72.
Henry, A. G., Brooks, A. S., and Piperno, D. R. 2011: Microfossils in Calculus Demonstrate Consumption of Plants and Cooked Foods in Neanderthal Diets (Shanidar III, Iraq; Spy I and II, Belgium). Proceedings of the National Academy of Sciences of the U.S.A. 108, 486–491.
Henry, A. G., Ungar, P. S., Passey, B. H., Sponheimer, M., Rossouw, L., Bamford, M., Sandberg, P., de Ruiter, D. J., and Berger, L. 2012: The Diet of Australopithecus sediba. Nature 487, 90–93.
Henry, A. G., Brooks, A. S., and Piperno, D. R. 2014: Plant Foods and the Dietary Ecology of Neanderthals and Early Modern Humans. Journal of Human Evolution 69, 44–54.
Hiscock, P., O’Connor, S., Balme, J., and Maloney, T. 2016: World’s Earliest Ground-Edge Axe Production Coincides with Human Colonisation of Australia. Australian Archaeology 82(1), 2–11.
Hoebler, C., Karinthi, A., Devaux, M. F., Guillon, F., Gallant, D. J., Bouchet, B., Melegari, C., and Barry, J. L. 1998: Physical and Chemical Transformations of Cereal Food During Oral Digestion in Human Subjects. British Journal of Nutrition 80, 429–436.
Hoffecker, J. F., Baryshnikov, G., and Potapova, O. 1991: Vertebrate Remains from the Mousterian Site of Il’skaya I (Northern Caucasus, U.S.S.R.): New Analysis and Interpretation. Journal of Archaeological Science 18, 113–147.
Hohmann, G., Potts, K., N’Guessan, A., Fowler, A., Mundry, R., Ganzhorn, J. U., and Ortmann, S. 2010: Plant Foods Consumed by Pan: Exploring the Variation of Nutritional Ecology across Africa. American Journal of Physical Anthropology 141, 476–485.
Inchley, C. E., Larbey, C. D. A., Shwan, N. A. A., Pagani, L., Saag, L., Antão, T., Jacobs, G., Hudjashov, G., Metspalu, E., Mitt, M., Eichstaedt, C. A., Malyarchuk, B., Derenko, M., Wee, J., Abdullah, S., Ricaut, F.-X., Mormina, M., Mägi, R., Villems, R., Metspalu, M., Jones, M. K., Armour, J. A. L., and Kivisild, T. 2016: Selective Sweep on Human Amylase Genes Postdates the Split with Neanderthals. Science Reports 6: 37198.
Ingicco, T., van den Bergh, G. D., Jago-on, C., Bahain, J.-J., Chacón, M. G., Amano, N., Forestier, H., King, C., Manalo, K., Nomade, S., Pereira, A., Reyes, M. C., Sémah, A.-M., Shao, Q., Voinchet, P., Falguères, C., Albers, P. C. H., Lising, M., Lyras, G., Yurnaldi, D., Rochette, P., Bautista, A., and de Vos, J. 2018: Earliest Known Hominin Activity in the Philippines by 709 Thousand Years Ago. Nature 557, 233–237.
Jaouen, K., Richards, M. P., Le Cabec, A., Welker, F., Rendu, W., Hublin, J.-J., Soressi, M., and Talamo, S. 2019: Exceptionally High δ15N Values in Collagen Single Amino Acids Confirm Neandertals as High-Trophic Level Carnivores. Proceedings of the National Academy of Science USA 116, 4928–4933.
Jones, M. 2009: Moving North: Archaeobotanical Evidence for Plant Diet in Middle and Upper Paleolithic Europe. In: J.-J. Hublin and M. P. Richards (eds.), The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht: Springer, 171–180.
Joordens, J. C. A., Wesselingh, F. P., de Vos, J., Vonhof, H. B., and Kroon, D. 2009: Relevance of Aquatic Environments for Hominins: A Case Study from Trinil (Java, Indonesia). Journal of Human Evolution 57, 656–671.
Kealy, S., O’Connor, S., Mahirta, Sari, D. M., Shipton, C., Langley, M. C., Boulanger, C., Kaharudin, H. A. F., Patridina, E. P. B. G. G., Algifary, M. A., Irfan, A., Beaumont, P., Jankowski, N., Hawkins, S., and Louys, J. 2020: Forty-Thousand Years of Maritime Subsistence Near a Changing Shoreline on Alor Island (Indonesia). Quaternary Science Reviews 249: 106599. https://doi.org/10.1016/j.quascirev.2020.106599.
Koebnick, C., Strassner, C., Hoffmann, I., and Leitzmann, C. 1999: Consequences of a Long-Term Raw Food Diet on Body Weight and Menstruation: Results of a Questionnaire Survey. Annals of Nutrition and Metabolism 43(2), 69–79.
Kotzamani, G. 2009: From Gathering to Cultivation: Archaeobotanical Research on the Early Plant Exploitation and the Beginning of Agriculture in Greece (Theopetra Schisto, Sidari, Dervenia). Thesis, University of Thessaloniki.
Kuhn, S. L. and Stiner, M. C. 2006: What’s a Mother to Do? The Division of Labor among Neandertals and Modern Humans in Eurasia. Current Anthropology 47, 953–980.
Laden, G. and Wrangham, R. 2005: The Rise of the Hominids as an Adaptive Shift in Fallback Foods: Plant Underground Storage Organs (USOs) and Australopith Origins. Journal of Human Evolution 49, 482–498.
Larbey, C., Mentzer, S. M., Ligouis, B., Wurz, S., and Jones, M. K. 2019: Cooked Starchy Food in Hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa. Journal of Human Evolution 131, 210–227.
Laville, H. and Renault-Miskovsky, J. (eds.) 1977: Approche écologique de l’homme fossile. Travaux du groupe: Ouest de l’Europe de la Commission Internationale de l’INQUA: Palecology of Early Man (1973-1977). Paris: Université Pierre et Marie Curie.
Lee-Thorp, J., Likius, A., Mackaye, H. T., Vignaud, P., Sponheimer, M., and Brunet, M. 2012: Isotopic Evidence for an Early Shift to C4 Resources by Pliocene Hominins in Chad. Proceedings of the National Academy of Sciences of the U.S.A. 109, 20369–20372.
Leppard, T. P. 2015: The Evolution of Modern Behaviour and Its Implications for Maritime Dispersal During the Palaeolithic. Cambridge Archaeological Journal 25, 829–846.
Lev, E., Kislev, M. E., and Bar-Yosef, O. 2005: Mousterian Vegetal Food in Kebara Cave, Mt. Carmel. Journal of Archaeological Science 32, 475–484.
Low, T. 1991: Wild Food Plants of Australia. North Ryde: Angus & Robertson Publishing.
Madella, M., Jones, M. K., Goldberg, P., Goren, Y., and Hovers, E. 2002: The Exploitation of Plant Resources by Neanderthals in Amud Cave (Israel): The Evidence from Phytolith Studies. Journal of Archaeological Science 29, 703–719.
Mandel, A. L. and Breslin, P. A. S. 2012: High Endogenous Salivary Amylase Activity is Associated with Improved Glycemic Homeostasis Following Starch Ingestion in Adults. The Journal of Nutrition 142, 853–858.
Mandel, A. L., Peyrot des Gachons, C., Plank, K. L., Alarcon, S., and Breslin, P. A. S. 2010: Individual Differences in AMY1 Gene Copy Number, Salivary α-Amylase Levels, and the Perception of Oral Starch. PLoS ONE 5(10): e13352.
Marean, C. W. and Assefa, Z. 1999: Zooarcheological Evidence for the Faunal Exploitation Behavior of Neandertals and Early Modern Humans. Evolutionary Anthropology 8, 22–37.
Martínez, K. 2006: Comments on ‘S. L. Kuhn and M. C. Stiner, What’s a Mother to Do? The Division of Labor among Neandertals and Modern Humans in Eurasia’. Current Anthropology 47, 967–968.
Matsutani, A. 1987: Plant Remains from the 1984 Excavations at Douara Cave. In: T. Akazawa and Y. Sakaguchi (eds.), Paleolithic Site of the Douara Cave and Paleogeography of Palmyra basin in Syria. Part IV: 1984 Excavations. Tokyo: University of Tokyo Press, 117–122.
McConnell, K. and O’Connor, S. 1997: 40,000 Year Record of Food Plants in the Southern Kimberley Ranges, Western Australia. Australian Archaeology 45, 20–31.
McPherron, S. P., Alemseged, Z., Marean, C. W., Wynn, J. G., Reed, D., Geraads, D., Bobe, R., and Béarat, H. A. 2010: Evidence for Stone-Tool-Assisted Consumption of Animal Tissues Before 3.39 Million Years Ago at Dikika, Ethiopia. Nature 466, 857–860.
Melamed, Y., Kislev, M. E., Geffen, E., Lev-Yadun, S., and Goren-Inbar, N. 2016: The Plant Component of an Acheulian Diet at Gesher Benot Ya’aqov, Israel. Proceedings of the National Academy of Sciences of the U.S.A. 113, 14674–14679.
Mercader, J. 2009: Mozambican Grass Seed Consumption During the Middle Stone Age. Science 326, 1680–1683.
Messager, E., Lordkipanidze, D., Ferring, C. R., and Deniaux, B. 2008: Fossil Fruit Identification by SEM Investigations, a Tool for Palaeoenvironmental Reconstruction of Dmanisi Site, Georgia. Journal of Archaeological Science 35, 2715–2725.
Milton, K. 1987: Primate Diets and Gut Morphology: Implications for Hominid Evolution. In: M. Harris and E. B. Ross (eds.), Food and Evolution. Toward a Theory of Human Food Habits. Philadelphia: Temple University Press, 93–115.
Milton, K. 1999: A Hypothesis to Explain the Role of Meat-Eating in Human Evolution. Evolutionary Anthropology 8, 11–21.
Milton, K. 2000: Hunter-Gatherer Diets – A Different Perspective. American Journal of Clinical Nutrition 71, 665–667.
Milton, K. 2003: The Critical Role Played by Animal Source Foods in Human (Homo) Evolution. American Society for Nutritional Sciences 133, 3886S–3892S.
Morley, M. W., Goldberg, P., Sutikna, T., Tocheri, M. W., Prinsloo, L. C., Jatmiko, Saptomo, E. W., Wasisto, S., and Roberts, R. G. 2017: Initial micromorphological results from Liang Bua, Flores (Indonesia): Site Formation Processes and Hominin Activities at the Type Locality of Homo floresiensis. Journal of Archaeological Science 77, 125–142.
Naito, Y. I., Chikaraishi, Y., Drucker, D. G., Ohkouchi, N., Semal, P., Wißing, C., and Bocherens, H. 2016: Ecological Niche of Neanderthals from Spy Cave Revealed by Nitrogen Isotopes of Individual Amino Acids in Collagen. Journal of Human Evolution 93, 82–90.
Naughton, J. M., O’Dea, K., and Sinclair, A. J. 1986: Animal Foods in Traditional Australian Aboriginal Diets: Polyunsaturated and low in fat. Lipids 21, 684–690.
Noli, D. and Avery, G. 1988: Protein Poisoning and Coastal Subsistence. Journal of Archaeological Science 15, 395–401.
O’Connell, J. F. and Allen, J. 2012: The Restaurant at the eEnd of the Universe: Modelling the Colonisation of Sahul. Australian Archaeology 74, 5–17.
O’Connell, J. F., Hawkes, K., and Blurton Jones, N. G. 1999: Grandmothering and the Evolution of Homo erectus. Journal of Human Evolution 36, 461–485.
O’Connell, J. F., Hawkes, K., Lupo, K. D., and Blurton Jones, N.G. 2002: Male Strategies and Plio-Pleistocene Archaeology. Journal of Human Evolution 43, 831–872.
O’Connor, S., Ono, R., and Clarkson, C. 2011: Pelagic Fishing at 42,000 Years Before the Present and the Maritime Skills of Modern Humans. Science 334, 1117–1121.
O’Connor, S., Robertson, G., and Aplin, K. P. 2014: Are Osseous Artefacts a Window to Perishable Material Culture? Implications of an Unusually Complex Bone Tool from the Late Pleistocene of East Timor. Journal of Human Evolution 67, 108–119.
O’Connor, S., Louys, J., Kealy, S., and Samper Carro, S. C. 2017: Hominin Dispersal and Settlement East of Huxley’s Line. Current Anthropology 58,Supplement 17, S567–S582.
Perera, N., Kourampas, N., Simpson, I. A., Deraniyagala, S. U., Bulbeck, D., Kamminga, J., Perera, J., Fuller, D. Q., Szabó, K., and Oliveira, N. V. 2011: People of the Ancient Rainforest: Late Pleistocene Foragers at the Batadomba-lena Rockshelter, Sri Lanka. Journal of Human Evolution 61, 254–269.
Perry, G. H., Dominy, N. J., Claw, K. G., Lee, A. S., Fiegler, H., Redon, R., Werner, J., Villanea, F. A., Mountain, J. L., Misra, R., Carter, N. P., Lee, C., and Stone, A. C. 2007: Diet and the Evolution of Human Amylase Gene Copy Number Variation. Nature Genetics 39, 1256–1260.
Peyrot des Gachons, C. and Breslin, P A. S. 2016: Salivary Amylase: Digestion and Metabolic Syndrome. Current Diabetes Reports 16(10): 102.
Pontzer, H. and Wood, B. M. 2021: Effects of Evolution, Ecology, and Economy on Human Diet: Insights from HunterGatherers and Other Small-Scale Societies. Annual Review of Nutrition 41, 363–385.
Potts, R. and Shipman, P. 1981: Cutmarks Made by Stone Tools on Bones from Olduvai George, Tanzania. Nature 291, 577–580.
Power, R. C. and L’Engle Williams, F. 2018: Evidence of Increasing Intensity of Food Processing During the Upper Paleolithic of Western Eurasia. Journal of Paleolithic Archaeology 1, 281–301.
Power, R. C., Salazar-García, D. C., Rubini, M., Darlas, A., Harvati, K., Walker, M., Hublin, J.-J., and Henry, A. G. 2018: Dental Calculus Indicates Widespread Plant Use within the Stable Neanderthal Dietary Niche. Journal of Human Evolution 119, 27–41.
Premathilake, R. and Hunt, C. O. 2018a: Earliest Musa Banana from the Late Quaternary Sequence at Fahien Rock Shelter in Sri Lanka. Journal of Quaternary Science 33, 624–638.
Premathilake, R. and Hunt, C. O. 2018b: Late Pleistocene Humans in Sri Lanka Used Plant Resources: A Phytolith Record from Fahien Rock Shelter. Palaeogeography, Palaeoclimatology, Palaeoecology 505, 1–17.
Prüfer, K., Racimo, F., Patterson, N., Jay, F., Sankararaman, S., Sawyer, S., Heinze, A., Renaud, G., Sudmant, P. H., de Filippo, C., Li, H., Mallick, S., Dannemann, M., Fu, Q., Kircher, M., Kuhlwilm, M., Lachmann, M., Meyer, M., Ongyerth, M., Siebauer, M., Theunert, C., Tandon, A., Moorjani, P., Pickrell, J., Mullikin, J. C., Vohr, S. H., Green, R. E., Hellmann, I., Johnson, P. L. F., Blanche, H., Cann, H., Kitzman, J. O., Shendure, J., Eichler, E. E., Lein, E. S., Bakken, T. E., Golovanova, L. V., Doronichev, V. B., Shunkov, M. V., Derevianko, A. P., Viola, B., Slatkin, M., Reich, D., Kelso, J., and Pääbo, S. 2014: The Complete Genome Sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49.
Richards, M. P. and Trinkaus. E. 2009: Isotopic Evidence for the Diets of European Neanderthals and Early Modern Humans. Proceedings of the National Academy of Sciences of the U.S.A. 106, 16034–16039.
Richards, M. P., Pettitt, P. B., Trinkaus, E., Smith, F. H., Paunović, M., and Karavanić, I. 2000: Neanderthal Diet at Vindija and Neanderthal Predation: The Evidence from Stable Isotopes. Proceedings of the National Academy of Sciences of the U.S.A. 97, 7663–7666.
Roberts, P., Perera, N., Wedage, O., Deraniyagala, S., Perera, J., Eregama, S., Petraglia, M. D., and Lee-Thorp, J. A. 2017: Fruits of the Forest: Human Stable Isotope Ecology and Rainforest Adaptations in Late Pleistocene and Holocene (~36 to 3ka) Sri Lanka. Journal of Human Evolution 106, 102–118.
Roberts, P., Louys, J., Zech, J., Shipton, C., Kealy, S., Carro, S. S., Hawkins, S., Boulanger, C., Marzo, S., Fiedler, B., Boivin, N., Mahirta, Aplin, K., and O’Connor; S. 2020: Isotopic Evidence for Initial Coastal Colonization and Subsequent Diversification in the Human Occupation of Wallacea. Nature Communications 11: 2068.
Robinson, B. W. and Wilson, D. S. 1998: Optimal Foraging, Specialization, and a Solution to Liem’s Paradox. American Naturalist 151, 223–235.
Roebroeks, W. and Villa, P. 2011: On the Earliest Evidence for Habitual Use of Fire in Europe. Proceedings of the National Academy of Sciences of the U.S.A. 108, 5209–5214.
Rothman, J. M., Dierenfeld, E. S., Hintz, H. F., and Pell, A. N. 2008: Nutritional Quality of Gorilla Diets: Consequences of Age, Sex, and Season. Oecologia 155, 111–122.
Rudman, D., DiFulco, T. J., Galambos, J. T., Smith III, R. B., Salam, A. A. and Warren, W. D. 1973: Maximal Rates of Excretion and Synthesis of Urea in Normal and Cirrhotic Subjects. The Journal of Clinical Investigation 52, 2241–2249.
Russell-Smith, J., Lucas, D., Gapindi, M., Gunbunuka, B., Kapirigi, N., Namingum, G., Lucas, K., Guiliani, P., and Chaloupka, G. 1997: Aboriginal Resource Utilization and Fire Management Practice in Western Arnhem Land, Monsoonal Northern Australia: Notes for Prehistory, Lessons for the Future. Human Ecology 25, 159–195.
Salazar-García, D. C., Power, R. C., Rudaya, N., Kolobova, K., Markin, S., Krivoshapkin, A., Henry, A. G., Richards, M. P., and Viola, B. 2021: Dietary Evidence from Central Asian Neanderthals: A Combined Isotope and Plant Microremains Approach at Chagyrskaya Cave (Altai, Russia). Journal of Human Evolution 156: 102985.
Samper Carro, S. C., O’Connor, S., Louys, J., Hawkins, S., and Mahirta 2016: Human Maritime Subsistence Strategies in the Lesser Sunda Islands During the Terminal Pleistocene–Early Holocene: New Evidence from Alor, Indonesia. Quaternary International 416, 64–79.
Samuelson, L. C., Phillips, R. S., and Swanberg, L. J. 1996: Amylase Gene Structures in Primates: Retroposon Insertions and Promoter Evolution. Molecular Biology and Evolution 13, 767–779.
Sandgathe, D. M. 2017: Identifying and Describing Pattern and Process in the Evolution of Hominin Use of Fire. Current Anthropology 58, Supplement 16, S360–S370.
Shahack-Gross, R., Berna, F., Karkanas, P., Lemorini, C., Gopher, A., and Barkai; R. 2014: Evidence for the Repeated Use of a Central Hearth at Middle Pleistocene (300 ky ago) Qesem Cave, Israel. Journal of Archaeological Science 44, 12–21.
Shipton, C., O’Connor, S., Jankowski, N., O’Connor-Veth, J., Maloney, T., Kealy, S., and Boulanger, C. 2019: A New 44,000-Year Sequence from Asitau Kuru (Jerimalai), Timor-Leste, Indicates Long-Term Continuity in Human Behaviour. Archaeological and Anthropological Sciences 11, 5717–5741.
Shipton, C., O’Connor, S., Kealy, S., Mahirta, Syarqiyah, I. N., Alamsyah, N., and Ririmasse, M. 2020: Early Ground Axe Technology in Wallacea: The First Excavations on Obi Island. PLoS ONE 15(8): e0236719.
Shipton, C., O’Connor, S., and Kealy, S. 2021: The Biogeographic Threshold of Wallacea in Human Evolution. Quaternary International 574, 1–12.
Sistiaga, A., Mallol, C., Galván, B., and Summons; R. E. 2014: The Neanderthal Meal: A New Perspective Using Faecal Biomarkers. PLoS ONE 9(6): e101045.
Sonestedt, E. 2018: Salivary Amylase Gene Variations Influence the Physiologic Response to Starchy Foods: 2 Sides of the Story. American Journal of Clinical Nutrition 108, 656–657.
Sorensen, A. C. 2019: The Uncertain Origins of Fire-Making by Humans: The State of the Art and Smouldering Questions. Mitteilungen der Gesellschaft für Urgeschichte 28, 11–50.
Speth, J. D. and Spielmann, K. A. 1983: Energy Source, Protein Metabolism, and Hunter-Gatherer Subsistence Strategies. Journal of Anthropological Archaeology 2, 1–31.
Stefansson, V. 1944: Arctic Manual. New York: Macmillan.
Stiner, M. C. 1994: Honor Among Thieves: A Zooarchaeoological Study of Neandertal Ecology. Princeton: Princeton University Press.
Stiner, M. C. 2001: Thirty Years on the “Broad Spectrum Revolution” and Paleolithic Demography. Proceedings of the National Academy of Sciences of the U.S.A. 98, 6993–6996.
Stiner, M. C. and Kuhn. S. L. 2009: Paleolithic Diet and the Division of Labor in Mediterranean Eurasia. In: J.-J. Hublin and M. P. Richards (eds.), The Evolution of Hominin Diets. Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht: Springer, 157–169.
Stringer, C. B., Finlayson, J. C., Barton, R. N. E., Fernández-Jalvo, Y., Cáceres, I. Sabin, R. C., Rhodes, E. J., Currant, A. P., Rodríguez-Vidal, J., Giles-Pacheco, F., and Riquelme-Cantal, J A. 2008: Neanderthal Exploitation of Marine Mammals in Gibraltar. Proceedings of the National Academy of Sciences of the U.S.A. 105, 14319–14324.
Summerhayes, G. R., Leavesley, M., Fairbairn, A., Mandui, H., Field, J., Ford, A., and Fullagar, R. 2010: Human Adaptation and Plant Use in Highland New Guinea 49,000 to 44,000 Years Ago. Science 330, 78–81.
Sutikna, T., Tocheri, M. W., Morwood, M. J., Saptomo, E. W., Jatmiko, Awe, R. D., Wasisto, S., Westaway, K. E., Aubert, M., Li, B., Zhao, J.-x., Storey, M., Alloway, B. V., Morley, M. W., Meijer, H. J. M., van den Bergh, G. D., Grün, R., Dosseto, A., Brumm, A., Jungers, W. L., and Roberts, R. G. 2016: Revised Stratigraphy and Chronology for Homo floresiensis at Liang Bua in Indonesia. Nature 532, 366–369.
Teixeira, J. C., Jacobs, G. S., Stringer, C., Tuke, J., Hudjashov, G., Purnomo, G. A., Sudoyo, H., Cox, M. P., Tobler, R., Turney, C. S. M., Cooper, A., and Helgen, K. M. 2021: Widespread Denisovan Ancestry in Island Southeast Asia but No Evidence of Substantial Super-Archaic Hominin Admixture. Nature Ecology and Evolution 5, 616–624.
Thieme, H. 1997: Lower Palaeolithic Hunting Spears from Germany. Nature 385, 807–810.
Tsartsidou, G., Karkanas, P. Marshall, G., and Kyparissi-Apostolika, N. 2014: Palaeoenvironmental Reconstruction and Flora Exploitation at the Palaeolithic Cave of Theopetra, Central Greece: The Evidence from Phytolith Analysis. Archaeological and Anthropological Sciences 7, 169–185.
Ungar, P. 2004: Dental Topography and Diets of Australopithecus afarensis and Early Homo. Journal of Human Evolution 46, 605–622.
van den Bergh, G. D., Meijer, H. J. M., Awe, R. D., Morwood, M. J., Szabó, K., van den Hoek Ostende, L. W., Sutikna, T., Saptomo, E. W., Piper, P. J., and Dobney, K. M. 2009: The Liang Bua Faunal Remains: A 95k.yr. Sequence from Flores, East Indonesia. Journal of Human Evolution 57, 527–537.
van den Bergh, G. D., Kaifu, Y., Kurniawan, I., Kono, R. T., Brumm, A., Setiyabudi, E., Aziz, F., and Morwood, M. J. 2016a: Homo floresiensis-Like Fossils from the Early Middle Pleistocene of Flores. Nature 534, 245–248.
van den Bergh, G. D., Li, B., Brumm, A., Grün, R., Yurnaldi, D., Moore, M. W., Kurniawan, I., Setiawan, R., Aziz, F., Roberts, R. G., Suyono, Storey, M., Setiabudi, E., and Morwood, M. J. 2016b: Earliest Hominin Occupation of Sulawesi, Indonesia. Nature 529, 208–211.
van Welzen, P. C., Parnell, J. A. N., and Ferry Slik, J. W. 2011: Wallace’s Line and Plant Distributions: Two or Three Phytogeographical Areas and Where to Group Java? Biological Journal of the Linnean Society 103, 531–545.
Vasil’ev, S. A., Kuzmin, Y. V., Orlova, L. A., and Dementiev, V. N. 2016: Radiocarbon-Based Chronology of the Paleolithic in Siberia and Its Relevance to the Peopling of the New World. Radiocarbon 44, 503–530.
Vogel, E. R., Alavi, S. E., Utami-Atmoko, S. S., van Noordwijk, M. A., Bransford, T. D., Erb, W. M., Zulfa, A., Sulistyo, F., Farida, W. R., and Rothman, J. M. 2017: Nutritional Ecology of Wild Bornean Orangutans (Pongo pygmaeus wurmbii) in a Peat Swamp Habitat: Effects of Age, Sex, and Season. American Journal of Primatology 79: e22618.
Wadley, L., Backwell, L., d’Errico, F., and Sievers, C. 2020: Cooked Starchy Rhizomes in Africa 170 Thousand Years Ago. Science 367, 87–91.
Watts, D. P. 2020: Meat Eating by Nonhuman Primates: A Review and Synthesis. Journal of Human Evolution 149: 102882.
Watts, D. P., Potts, K. B., Lwanga, J. S., and Mitani, J. C. 2012: Diet of Chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda, 1. Diet Composition and Diversity. American Journal of Primatology 74, 114–129.
Wedage, O., Amano, N., Langley, M. C., Douka, K., Blinkhorn, J., Crowther, A., Deraniyagala, S., Kourampas, N., Simpson, I., Perera, N., Picin, A., Boivin, N., Petraglia, M., and Roberts, P. 2019a: Specialized Rainforest Hunting by Homo sapiens ~45,000 Years Ago. Nature Communications 10: 739.
Wedage, O., Picin, A., Blinkhorn, J., Douka, K., Deraniyagala, S., Kourampas, N., Perera, N., Simpson, I., Boivin, N., Petraglia, M., and Roberts, P. 2019b: Microliths in the South Asian Rainforest ~45-4 ka: New Insights from Fa-Hien Lena Cave, Sri Lanka. PLoS ONE 14(10): e0222606.
Wedage, O., Roberts, P., Faulkner, P., Crowther, A., Douka, K., Picin, A., Blinkhorn, J., Deraniyagala, S., Boivin, N., Petraglia, M., and Amano, N. 2020: Late Pleistocene to Early-Holocene Rainforest Foraging in Sri Lanka: Multidisciplinary Analysis at Kitulgala Beli-lena. Quaternary Science Reviews 231: 106200.
Weyrich, L. S., Duchene, S., Soubrier, J., Arriola, L., Llamas, B., Breen, J., Morris, A. G., Alt, K. W., Caramelli, D., Dresely, V., Farrell, M., Farrer, A. G., Francken, M., Gully, N., Haak, W., Hardy, K., Harvati, K., Held, P., Holmes, E. C., Kaidonis, J., Lalueza-Fox, C., de la Rasilla, M., Rosas, A., Semal, P., Soltysiak, A., Townsend, G., Usai, D., Wahl, J., Huson, D. H., Dobney, K., and Cooper, A. 2017: Neanderthal Behaviour, Diet, and Disease Inferred from Ancient DNA in Dental Calculus. Nature 544, 357–361.
Will, M., Kandel, A. W., and Connard, N. J. 2019: Midden or Molehill: The Role of Coastal Adaptations in Human Evolution and Dispersal. Journal of World Prehistory 32, 33–72.
Wißing, C., Rougier, H., Crevecoeur, I., Germonpré, M., Naito, Y. I., Semal, P., and Bocherens, H. 2016: Isotopic Evidence for Dietary Ecology of Late Neandertals in North-Western Europe. Quaternary International 411, 327–345.
Wood, B. and Collard, M. 1999a: The Changing Face of the Genus Homo. Evolutionary Anthropology 8, 195–207.
Wood, B. and Collard, M. 1999b: The Human Genus. Science 284, 65–71.
Wrangham, R. W. 2007: The Cooking Enigma. In: P. S. Ungar (ed.), Evolution of the Human Diet: The Known, the Unknown and the Unknowable. Oxford: Oxford University Press, 308–323.
Wrangham, R. 2017: Control of Fire in the Paleolithic. Evaluating the Cooking Hypothesis. Current Anthropology 58, Supplement 16, S303–S313.
Wrangham, R. and Carmody, R. 2010: Human Adaptation to the Control of Fire. Evolutionary Anthropology 19, 187–199.
Wrangham, R. W., Jones, J. H., Laden, G., Pilbeam, D., and Conklin-Brittain, N. 1999: The Raw and the Stolen. Cooking and the Ecology of Human Origins. Current Anthropology 40, 567–594.
Zilhão, J., Angelucci, D. E., Araújo Igreja, M., Arnold, L. J., Badal, E., Callapez, P., Cardoso, J. L., d’Errico, F., Daura, J., Demuro, M., Deschamps, M., Dupont, C., Gabriel, S., Hoffmann, D. L., Legoinha, P., Matias, H., Monge Soares, A. M., Nabais, M., Portela, P., Queffelec, A., Rodrigues, F., and Souto, P. 2020: Last Interglacial Iberian Neandertals as Fisher-Hunter-Gatherers. Science 367: eaaz7943.
Zink, K. D. and Lieberman, D. E. 2016: Impact of Meat and Lower Palaeolithic Food Processing Techniques on Chewing in Humans. Nature 531, 500–503.