Abstract
Knee osteoarthritis is commonly thought to be caused by joint tissue wear and tear produced by physical activity. Activities that subject knees to repetitive impacts characterized by high rates of loading are believed to be especially harmful. Here, we present an alternative hypothesis that physical activity, rather than necessarily being bad for knee tissues, may help prevent or attenuate knee osteoarthritis, including activities involving high rates of loading. We experimentally tested this hypothesis using guinea pigs as a model system. To simulate a physically inactive lifestyle, animals were housed for 22 weeks in small cages that restricted their mobility, while two other groups of animals were housed in one of two large rooms that promoted physical activity. One room had a stiff floor to engender high rates of hind limb loading, whereas the floor in the other room was cushioned to engender low rates of hind limb loading. After the experiment, we found that knee osteoarthritis degeneration was significantly greater among the physically inactive animals than among the physically active animals in both the stiff- and cushioned-floored rooms. These results support our hypothesis and challenge common assumptions about the effects of physical activity and impact loading rate on knee osteoarthritis.
Introduction
Knee osteoarthritis (OA) is a debilitating disease involving articular cartilage degeneration coupled with changes in nearby bone and synovial tissue. A common perception of knee OA pathophysiology is that cartilage degeneration is caused by the accumulation of wear and tear engendered by physical activity throughout life (Brandt et al. 2009; Jurmain 1977; Radin et al. 1972, 1991; Turner et al. 2007). Among the activities expected to be most harmful are those that expose knees to repetitive impacts characterized by high rates of loading (Brandt et al. 2009; Radin 2004). These may include athletic activities such as long-distance running or football (Driban et al. 2017), as well as everyday activities like walking on stiff ground surfaces (e.g., concrete pavement) or in stiff-soled shoes (Lafortune and Hennig 1992; Radin et al. 1982; Whittle 1999). Thus, it has been suggested that to prevent knee OA or delay disease progression, activities producing high rates of knee loading should generally be avoided, or at least take place on soft ground surfaces (Milgrom et al. 1998, 2003), and shoes with cushioned soles should be worn (Fernandes et al. 2013; McAlindon et al. 2014; Paterson et al. 2014).
Recently, we have challenged this wear and tear perception of knee OA by hypothesizing that physical activity, instead of being inevitably bad for knee health, may actually help prevent or attenuate knee OA degeneration (Berenbaum et al. 2018; Wallace et al. 2017, 2019, 2022), including activities involving high rates of loading (Holowka et al. 2021; Wallace et al. 2018). This hypothesis originated based primarily on clues from human evolution. For >95% of human evolution, all people were hunter-gatherers with physically active lifestyles that necessitated walking long distances on a daily basis (Kraft et al. 2021; Marlowe 2005), and under certain conditions, also frequent long-distance running (Bramble and Lieberman 2004; Carrier 1984). In addition, for the vast majority of that time, our ancestors exclusively walked barefoot, which is known to generate ground reaction forces much more rapidly than walking in shoes, regardless of shoe sole stiffness (Holowka et al. 2019; Lafortune and Hennig 1992; Wallace et al. 2018). Yet, analyses of skeletal remains of ancient hunter-gatherers indicate that knee OA was much less prevalent in the past than today, even after accounting for variation in lifespan (Wallace et al. 2017). Given that knee OA levels are higher today than in the past, while average physical activity levels are now much lower and cushion-soled shoes are ubiquitous, it is reasonable to hypothesize that frequent high-rate impact loading may be beneficial, rather than detrimental, for knee health.
Despite evolutionary reasons to hypothesize that physical activity producing high-rate impacts may be less of a risk for knee OA than commonly believed, direct evidence supporting this hypothesis is currently limited. We therefore conducted an experiment to test potential links between physical activity, impact loading rate, and knee OA using guinea pigs as a model system. Guinea pigs are a suitable model because, like most humans who get knee OA, they develop the disease idiopathically, which makes them appropriate for testing potential inhibitors of knee OA degeneration (Bendele 2001). Moreover, previous studies have demonstrated that knee OA in guinea pigs is histopathologically similar to that of humans (Bendele and Hulman 1988; Kraus et al. 2010). In our experiment, to simulate a physically inactive lifestyle (which we hereafter refer to as “sedentary”), growing animals were individually housed for 22 weeks in small cages that restricted (but did not eliminate) their mobility, while two other groups of animals were group-housed in one of two large rooms that facilitated voluntary engagement in physical activity. One room had a stiff concrete floor to promote high rates of hind limb loading during locomotion, whereas the floor in the other room was covered with foam cushioning to promote low rates of hind limb loading. Our two hypotheses were that compared to sedentary animals, both groups of physically active animals would experience less knee OA degeneration throughout the experiment, and animals who were physically active on the stiff floor would experience no more knee OA degeneration than animals who moved on the cushioned floor. Data from the sedentary group and physically active group housed in the cushioned-floored room have been reported previously (Wallace et al. 2022).
Materials & Methods
Experimental design
All procedures were approved by the IACUC of Harvard University. Male Hartley guinea pigs (n=45) were acquired from Charles River Laboratories (Wilmington, MA, USA) at 7 weeks of age. Animals were randomly divided into a sedentary group and two physically active groups (n=15/group). Sedentary animals were housed individually in small cages (width × length: 27 × 48 cm) with wood-shavings bedding. Physically active animals were group-housed in one of two large rooms (width × length: 183 × 244 cm) with different types of flooring. One group was housed in a room with a stiff, epoxy-coated concrete floor, and the other group was housed in a room with the same concrete floor covered with foam cushion flooring material (thickness: 11 mm, Young’s modulus: 1.6 MPa; Eco-Soft Plus tiles, Rubber Flooring Inc., Mesa, AZ, USA). Both rooms had a ceiling-mounted HDCVI camera to record physical activity (model: CSP-CVIED2-B, CCTV Security Pros, Cherry Hill, NJ, USA). The floors of both rooms were scrubbed and cleaned daily, which took approximately 30 min per room to complete, during which time the animals were kept in large plastic bins with wood-shavings bedding. The foam cushion flooring material was replaced at least every four weeks. All animals were kept on a 12:12-hr light/dark cycle, at an ambient temperature of approximately 25°C, with free access to water and food (LabDiet 5025, PMI Nutrition, St. Louis, MO, USA). At the age of 30 weeks, all animals were euthanized and right articulated knees were extracted and placed in 10% NBF for later histopathological analyses of knee OA degeneration.
The use of Hartley guinea pigs aged 7 to 30 weeks is suitable for studying the degree to which physical activity attenuates knee OA, since it is during this ontogenetic interval that idiopathic disease onset typically occurs, with initial histological signs present on the medial tibial plateau between 12 and 16 weeks of age (Bendele et al. 1989; Kraus et al. 2010). Importantly, however, OA degeneration in the medial femur and lateral knee compartment usually appears later in ontogeny and is less severe than that in the medial tibia (Bendele and Hulman 1988). Thus, this experiment was designed specifically to examine the effects of physical activity on knee OA onset rather than more advanced stages of the disease, and our analyses of OA degeneration focused on just the medial tibia.
Tibial histopathology
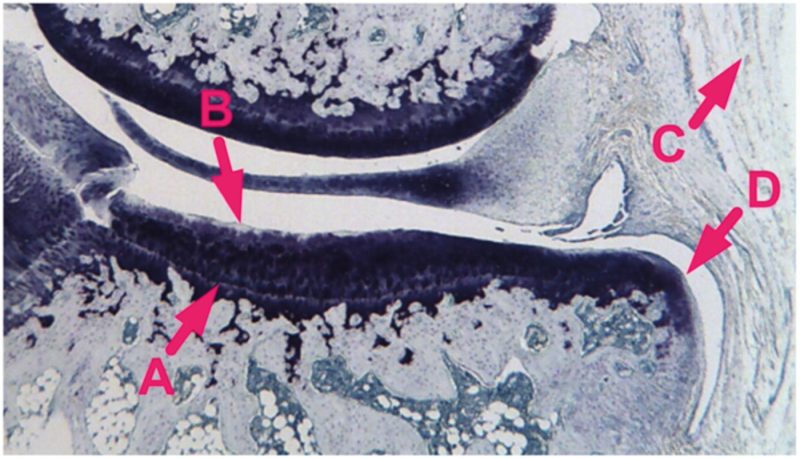
Right knee joints were decalcified for 10 days in 10% formic acid and embedded in paraffin in a slightly flexed position (Kraus et al. 2010). Two 8-μm coronal sections of the medial compartment were prepared, one anterior and one posterior, and then stained with toluidine blue (Fig. 1). Tibial histopathological assessments were performed blinded on the sections at a magnification of 25× using an ocular micrometer. Cartilage thickness (defined as depth to tidemark) was measured at the mediolateral midpoint of cartilage width (defined as the total mediolateral span of cartilage across the load-bearing surface of the medial tibial plateau). Cartilage width was divided into three equal-diameter zones (medial, central, and lateral) and cartilage degeneration in each zone was scored following an established method (Bendele et al. 1996). Scores were based on the evaluation of chondrocyte death/loss, matrix fibrillation/loss, and aggrecan loss, with chondrocyte loss being the main determinant of the scores. Degeneration in each zone was scored on a scale from 0 to 5 (none to severe), and a 3-zone-sum for degeneration was calculated by adding the values obtained for each zone. Following an established method (Bendele et al. 1996), synovial inflammation was scored on a scale from 0 to 5 (none to severe) based on the presence or absence of an increased number of synovial lining cell layers and proliferation of the subsynovial tissue. The thickness of osteophytes, when present, was measured from the tidemark to the furthest point extending toward the synovium. Values from the anterior and posterior sections were averaged.
Physical activity measurements
Among animals housed in the cushioned- and stiff-floored rooms, ceiling-mounted cameras were used to quantify physical activity levels on 5 separate days throughout the experiment. Cameras recorded at a rate of 5 frames per second during the 12 hours of each day when the lights were on. Room cleaning on these days took place when the lights were off. To help track animal movement, red circles were painted on the backs of animals using a non-toxic marker (Stoelting Co., Wood Dale, IL, USA). Before the beginning of the experiment, camera lens distortion coefficients were calculated based on the distortion of a checkerboard pattern held at different angles to the cameras. Additionally, a rod of known distance was placed on the ground at different angles to calibrate room dimensions in the camera recordings. For each 12-hour recording, the movement of each animal was automatically tracked from frame to frame using DLTdv software (Hedrick 2008). From these data, the average total distance traveled by the animals in each room during the 12-hour period was calculated for each of the 5 days.
Hind limb loading rate on cushioned versus stiff surfaces
To verify that the foam cushion flooring material had the predicted effect of decreasing the rate of hind limb loading, the vertical component of ground reaction forces generated by guinea pig locomotion was measured using a custom-built force plate, with and without a piece of the foam material covering the plate surface. The plate consisted of a load cell (Nano 43, ATI, Apex, NC, USA) housed within a 3D-printed chassis and a top plate (width × length: 15 × 15 cm) made of carbon fiber reinforced nylon. The plate was situated at the center of a plywood trackway (width × length: 15 × 400 cm). To collect data, 10 guinea pigs were randomly selected from the physically active cohorts. During trials, each animal was released at one end of the trackway and moved at a self-selected speed down the trackway and across the plate surface. Videos recorded in lateral view were used to identify trials resulting in single hind limb contacts of the load cell during steady-state locomotion. Animal speed was determined from videos as the time required for a fixed anatomical landmark (the nose) to pass between markers on either side of the trackway. Ground reaction force data were collected at 4 kHz and imported into Igor Pro software (v7.1, WaveMetrics Inc., Lake Oswego, OR, USA) via an analog-to-digital converter (USB-6521, National Instruments, Austin, TX, USA) and filtered with smoothing spline interpolation (smoothing factor≤0.05). Data from 10 hind limb contacts were collected with the foam material covering the plate surface and 10 hind limb contacts without the foam material. For each trial, the linear rise of the ground reaction force was measured using Igor Pro software to calculate hind limb loading rate. Values were normalized to body weight to facilitate comparisons across individuals.
Tibial strain rate on cushioned versus stiff surfaces
To further verify that the foam cushion flooring material decreased the rate of hind limb loading, an additional male Hartley guinea pig (aged 10 weeks) was purchased and used to measure tibial diaphyseal strain rates during locomotion on a treadmill with and without the foam material covering the treadmill belt surface. Under isoflurane general anesthesia, a single-element strain gauge (Sokki Kenkyujo, Tokyo, Japan) was affixed to the anterior surface of the left tibial mid-diaphysis of the animal. At the gauge site, a small skin incision was made to gain access to the bone, an area (2 × 2 mm) of the periosteum was elevated, the bone surface was degreased with chloroform, and the gauge was glued to the prepared surface with cyanoacrylate. Care was taken to align the gauge element with the long axis of the bone. Gauge leads were passed subcutaneously and emerged through a small skin incision on the animal’s back. Incisions were sutured closed. Strain data were recorded 12 hours after surgery while the animal trotted (30 m/s) on a level motorized treadmill (Woodway, Waukesha, WI, USA) with a belt composed of stiff, rubber-coated steel slats (0.56 × 0.07 m). Voltage changes in strain gauges were conditioned and amplified (Vishay 2150, MicroMeasurements Inc., Raleigh, NC, USA), and data were acquired through a DAQ board (PowerLab, ADInstruments, Colorado Springs, CO, USA) run by LabChart software (ADInstruments). Data were recorded from 10 gait cycles with the animal moving on the treadmill with strips of the foam cushion material attached to the belt slats, and from 10 gait cycles without the foam cushion material. For each gait cycle, the strain rate was calculated using Igor Pro software.
Statistics
Shapiro-Wilk tests were used to determine if data followed a normal distribution, and Levene’s tests were used to assess the equality of group variances. Statistical evaluation of differences among animals assigned to the sedentary group and those housed in the cushioned- and stiff-floored rooms was conducted with an analysis of variance (ANOVA) followed by a Tukey’s honestly significant difference (HSD) multiple comparisons test. When the equal variances assumption was violated, a Games-Howell (GH) multiple comparisons test was carried out. A general linear mixed model (GLMM) was used to compare hind limb loading rates (in units of body weight) during locomotion across the force plate with and without foam cushioning covering the plate surface, with animal speed included as a covariate, and animal identity and housing condition (cushioned- or stiff-floored room) included as random effects. Independent-samples t-tests were used to assess differences in tibial diaphyseal strain rates during locomotion on the treadmill with and without foam cushioning attached to the treadmill belt surface, as well as average daily movement distances between animals housed in the cushioned- versus stiff-floored rooms. Statistical analyses were conducted using JMP Pro software (v. 15, SAS Inst., Cary, NC, USA) and SPSS software (v. 20; IBM Corp., Armonk, NY, USA). Statistical significance was judged using a 95% criterion (P≤0.05), and tests were two-tailed.
Results
Effects of surface cushioning on hind limb biomechanics
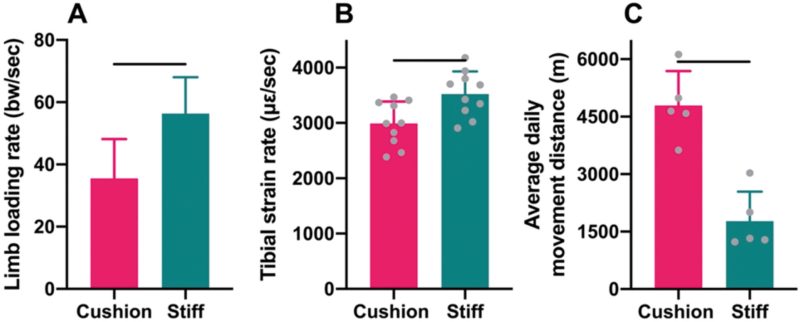
Guinea pig locomotion across the force plate with the foam cushion material attached to the plate surface engendered hind limb loading rates that were, on average, 37% lower than those produced during locomotion on a stiff, uncushioned force plate, after controlling for self-selected locomotor speeds (GLMM: P=0.035; Fig. 2A). Tibial diaphyseal strain rates generated during locomotion at a fixed speed on the treadmill with the foam cushion material attached to the belt surface were, on average, 15% lower compared to locomotion at the same speed on a stiff, uncushioned treadmill belt (t-test: P<0.01; Fig. 2B).
Physical activity levels
Throughout the experiment, animals housed in the rooms with cushioned and stiff floors both voluntarily engaged in higher levels of physical activity than possible among the sedentary animals housed in small, restrictive cages (Fig. 2C). The minimum and maximum average daily (12-hr) movement distances measured among animals in either room were 1.23 km and 6.12 km, respectively. Nevertheless, the activity levels of animals in the two different rooms were distinct. Specifically, the average daily movement distances of animals in the cushioned-floored room were 170% greater than those of animals in the stiff-floored room (t-test: P<0.001).
Body size
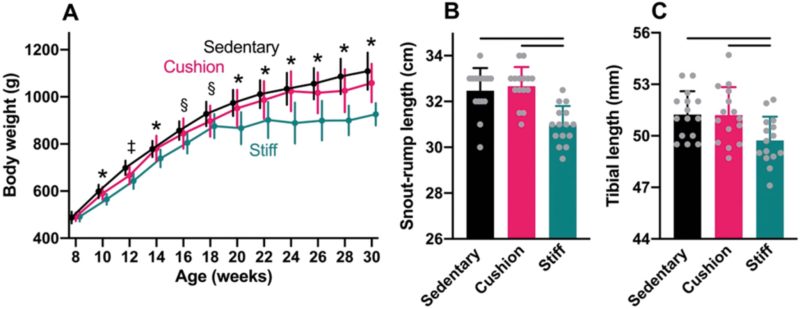
At the start of the experiment, average body weight was similar among animals assigned to the sedentary group and those housed in the cushioned- and stiff-floored rooms (ANOVA: P=0.88; Fig. 3A). Throughout the experiment, body weights increased at a similar rate among animals in the sedentary group and physically active animals in the cushioned-floored room, such that by the end of the experiment, average body weight did not differ significantly between the two groups (HSD: P=0.14). However, among physically active animals in the stiff-floored room, body weights increased markedly less during the experiment than among animals in the other two groups, especially after 18 weeks of age. By the end of the experiment, average body weight among animals in the stiff-floored room was 17% and 13% lower than among sedentary animals and those in the cushioned-floored room, respectively (HSD: P<0.0001 for both comparisons).
At the end of the experiment, average snout-to-rump length did not differ significantly between animals in the sedentary group and physically active animals in the cushioned-floored room (HSD: P=0.81; Fig. 3B), nor did average tibial length (HSD: P=0.99; Fig. 3C). Among physically active animals in the stiff-floored room, however, average snout-to-rump length was 5% less than among animals in both the sedentary group and those in the cushioned-floored room (HSD: P≤0.0001 for both comparisons). The average tibial length was 3% shorter among animals in the stiff-floored room, compared to animals in both of the other two groups (HSD: P=0.020 and P=0.024 for sedentary and cushioned-floored group comparisons, respectively).
Knee OA degeneration
In the medial tibia, at the end of the experiment, sedentary and physically active animals in the cushioned- and stiff-floored rooms presented cartilage of similar thickness (ANOVA: P=0.58; Fig. 4A). However, cartilage degeneration scores were significantly higher among sedentary animals than among physically active animals in both the cushioned- and stiff-floored rooms (GH: P=0.049 and P=0.029, respectively; Fig. 4B), as were synovial inflammation scores (GH: P=0.019 for both comparisons; Fig. 4C). Osteophytes were present in 53% (8/15) of sedentary animals and 67% (10/15) and 47% (7/15) of physically active animals in the cushioned- and stiff-floored rooms, respectively. Osteophytes among sedentary animals were, on average, 92% and 121% larger than those of physically active animals in the cushioned- and stiff-floored rooms, respectively (HSD: P<0.001 for both comparisons; Fig. 4D). No significant differences were detected between the two physically active groups in cartilage degeneration scores (GH: P=0.79), synovitis scores (GH: P=0.99), or osteophyte size (HSD: P=0.89).

Discussion
In this study, to assess the effects of physical activity and impact loading rate on knee OA degeneration, growing guinea pigs were raised for 22 weeks in one of three groups. A group of sedentary animals was housed in small cages that restricted mobility, and two groups of animals were housed in one of two large rooms that promoted voluntary physical activity. One room had a stiff concrete floor to engender high rates of hind limb loading during locomotion, and the other room had a floor covered with foam cushioning to engender lower rates of hind limb loading. Measurements of hind limb ground reaction forces and tibial bone strains confirmed that the cushion flooring material had the intended effect of generally lowering rates of hind limb loading. Our first hypothesis was that relative to sedentary animals, both groups of physically active animals would undergo less knee OA degeneration during the experiment. The results support this hypothesis. At the end of the experiment, compared to sedentary animals, both groups of physically active animals had significantly lower knee cartilage degeneration scores, lower synovial inflammation scores, and smaller osteophytes when present. Our second hypothesis was that between the two physically active groups, animals in the stiff-floored room would experience no more knee OA degeneration than animals housed in the cushioned-floored. The results also support this hypothesis. The two physically active groups had similar knee cartilage degeneration scores, synovial inflammation scores, and sizes of osteophytes when present. Overall, the results of this study provide support for the idea that physical activity, instead of being inevitably bad for knee health, has the potential to attenuate knee OA degeneration (Berenbaum et al. 2018; Griffin et al. 2012; Otterness et al. 1998; Wallace et al. 2017, 2019, 2022), including activities involving high rates of loading (Holowka et al. 2021; Wallace et al. 2018).
The precise pathways by which physical activity might attenuate knee OA degeneration are not fully known, but there are at least two possibilities. First, physical activity might help prevent the accumulation of excess body weight, a well-known risk factor for knee OA (Bendele and Hulman 1991; Felson et al. 1988; Wluka et al. 2013). Excess body weight likely affects knee OA degeneration by producing a combination of adiposity-induced metaflammation and abnormal joint loading (Berenbaum et al. 2018; Zapata-Linares et al. 2021). Throughout most of our experiment, physically active animals in the stiff-floored room had significantly lower body weight than sedentary animals, which may have contributed to their lower levels of knee OA degeneration. Differences in body weight, however, do not clearly explain differences in knee OA degeneration between physically active animals in the cushioned-floor room and sedentary animals since body weights were generally similar between the two groups. Though, it is possible that sedentary animals still had greater adiposity and hence adiposity-induced metaflammation. Second, physical activity while young may help promote the growth of stronger knee tissues that are more resistant to OA degeneration later in life (Helminen et al. 2000). Specifically, previous experiments have shown that young animals treated with exercise develop thicker knee cartilage, higher cartilage aggrecan content, and increased cartilage stiffness (Jurvelin et al. 1986; Kiviranta et al. 1988; Säämänen et al. 1988, 1989). In our study, cartilage thickness was found to be similar among animals in all three groups at the end of the experiment, but it is possible that there were important differences in knee tissue development between physically active and sedentary animals that we did not measure. Ultimately, additional research is necessary to better understand the pathways that underlie the benefits of physical activity for attenuating knee OA degeneration.
During knee loading, cartilage and subchondral bone undergo deformation, which helps to minimize stresses within the cartilage matrix. Because knee cartilage and subchondral bone are viscoelastic tissues, less deformation is expected to occur when loads are generated more rapidly. It is for this reason that physical activities exposing knees to high rates of loading have been hypothesized to be especially likely to cause cartilage damage and OA degeneration (Brandt et al. 2009; Radin 2004). This hypothesis, while sensible, is not supported by our finding that the physically active animals in the cushioned- and stiff-floored rooms had similarly low levels of knee OA degeneration relative to sedentary animals, despite animals in the stiff-floored room experiencing generally higher rates of hind limb loading. Also inconsistent with this hypothesis are the results of many previous studies showing that habitual engagement in activities producing high rates of knee loading (e.g., long-distance running) is not a strong predictor of knee OA risk (Lo et al. 2017; Newton et al. 1997; Timmins et al. 2017). Although some prior animal experiments provide support for the idea that higher rates of loading can be more damaging to joint tissues, these studies involved artificial loads applied under non-physiologic conditions (Ewers et al. 2002; Radin et al. 1985; Yang et al. 1989). In the only experiment that we are aware of besides our own that investigated the effects of higher versus lower rates of natural loading on knee tissues under physiologic conditions, sheep were forced to walk for 4 hr per day for 2.5 yr on either a stiff concrete surface or a compliant wood chip surface (Radin et al. 1982). At the end of the experiment, none of the animals in either group were found to have major signs of OA degeneration in any of their limb joints. These results, together with our own, suggest that knee cartilage and other tissues are adapted to withstand loads applied both rapidly and gradually, as long as loading is within the physiologic range.
Two findings that were unanticipated in our study are the marked differences in voluntary physical activity levels and body weights between animals in the cushioned- and stiff-floored rooms. When we noticed these differences during the experiment, we suspected they might have been due to temperature differences between the cushioned and stiff floors. To assess this idea, we measured room floor temperatures using a laser infrared thermometer gun (Lasergrip 1080, Etekcity Corp., Anaheim, CA, USA) on 5 days during the last 4 weeks of the experiment. On all days, ambient temperatures in both rooms were approximately 25°C, but the average floor temperatures in the cushioned- and stiff-floored rooms were 21.2°C and 17.3°C, respectively (t-test: P<0.001). The lower critical temperature of the guinea pig thermoneutral zone is approximately 20°C (Gordon 1986), and previous studies have shown that rodents raised at temperatures below their lower critical temperature can exhibit both decreased physical activity levels and body weights, as well as other traits that were ultimately found to be characteristic of animals raised in the stiff-floored room, including shorter limbs and body lengths (Chevillard et al. 1963; Robbins et al. 2018; Serrat 2014; Vaanholt et al. 2007). Thus, it seems likely that cooler floor temperatures contributed to the lower physical activity levels and body weights of the animals in the stiff-floored room.
Regardless of the exact causes of differences in physical activity and body weight between animals in the cushioned- and stiff-floored rooms, it is important to consider how such differences might affect interpretations of our results related to knee OA. Importantly, our first hypothesis, that physical activity has the potential to attenuate knee OA degeneration, is well supported by comparisons between the sedentary animals and physically active animals in the cushioned-floored room (Wallace et al. 2022). Moreover, even given their lower physical activity levels and body weights compared to animals in the cushioned-floored room, animals in the stiff-floored room were still more physically active than sedentary animals. Thus, our first hypothesis is also supported by comparisons between the sedentary animals and physically active animals in the stiff-floored room. However, in terms of our second hypothesis, that higher rates of activity-induced loading do not cause more knee OA degeneration than lower rates of loading, we cannot rule out the possibility that had physical activity levels and body weights been more alike between animals in the cushioned- and stiff-floored rooms, then levels of knee OA degeneration would not have been as similar between the two physically active cohorts. To rigorously evaluate this possibility, a more controlled experiment will need to be conducted in the future. Nevertheless, we maintain that none of our findings related to knee OA degeneration are inconsistent with our second hypothesis, nor are any results supportive of the common view that routine physical activities that expose knees to high rates of loading are especially harmful to knee tissues.
Another finding that deserves consideration is that physical activity decreased knee OA degeneration but did not prevent the disease altogether, either among animals in the cushioned- or stiff-floored rooms. Similar results were obtained in a previous study in which we investigated the effects of daily treadmill running on Hartley guinea pig knees and found that running reduced knee OA degeneration but did not totally inhibit the disease (Wallace et al. 2019). At the time of that study, we interpreted the finding as likely being due to the modest size of the exercise dosage that the runners received, amounting to only 3% of total time per day. We hypothesized that larger doses of physical activity might prevent knee OA outright. The results of the current study do not support this hypothesis, particularly the results from the physically active animals housed in the cushioned-floored room. Compared to the treadmill runners in our previous study, animals in the cushioned-floored room took, on average, roughly 4 times more steps per day, yet they still experienced some knee OA degeneration. In retrospect, it seems possible that vulnerability to knee OA is so high among Hartley guinea pigs that no dosage of physical activity (or any other potentially prophylactic action) could entirely prevent the disease (Bendele and Hulman 1988; Brismar et al. 2003; Hyttinen et al. 2001). Indeed, compared to other laboratory stocks of guinea pigs, Hartley guinea pigs have been shown to be much more susceptible to knee OA degeneration even when kept under identical environmental conditions (Huebner et al. 2002). If knee OA degeneration is basically inevitable among Hartley guinea pigs, then it may be better for future experimental studies of the preventative potential of physical activity to employ an alternative model system. Even so, regardless of why physical activity failed to totally prevent knee OA in our two experiments, both studies provide direct evidence that routine engagement in physical activity can at least attenuate knee OA degeneration.
From an evolutionary perspective, the results of this study are important because they provide additional support to the idea that knee OA represents an example of a ‘mismatch disease’ that is caused, in part, by the musculoskeletal system being poorly adapted to environmental factors that were once rare but now common, including excessively sedentary lifestyles (Berenbaum et al. 2018; Wallace et al. 2017). In contrast to laboratory guinea pigs, wild guinea pigs rarely experience knee OA degeneration (Rothschild 2003). Our findings suggest that this is likely partly because guinea pigs evolved to engage in higher levels of physical activity than laboratory animals are normally allowed (Zipser et al. 2014), and probably on surfaces of variable stiffness, hence their knees evolved to require routine physical activity to develop and function optimally and remain healthy. Since human musculoskeletal biology evolved among ancient physically active hunter-gatherers, human knees also presumably evolved to require and benefit from frequent loading, including high-rate knee loading like that engendered by walking long distances barefoot. However, even if knee OA is a mismatch disease, it would not cease to exist even if every person and guinea pig in the world adjusted their physical activity levels to more closely match those of their ancient ancestors. Trauma and other risk factors for knee OA have and will always predispose some individuals to the disease. Nevertheless, our results suggest that habitual engagement in physical activity may be a powerful strategy for attenuating knee OA degeneration, and that sedentism may be a greater threat to knee health than is often assumed.
Finally, the results of this study are relevant to anthropological studies of knee OA among fossil and archeological human skeletons. Based on the traditional view of knee OA as being caused by wear and tear produced by physical activity, many anthropologists have assumed that signs of knee OA in ancient human skeletons can be interpreted as evidence of a lifestyle characterized by high levels of physical activity (e.g., Austin 2017; Bridges 1991; Cheverko and Bartelink 2017; Jurmain 1977, 1999; Klaus et al. 2009; Larsen 1982, 2015; Larsen et al. 1995; Lieverse et al. 2007, 2016). The findings of this study highlight that not all types of physical activity should be assumed to be associated with greater knee OA degeneration, including everyday activities like walking and running that produce most of the activity-related loads that knees normally experience. Therefore, determining whether or not a person’s skeletal remains exhibit signs of knee OA is almost certainly an inaccurate way of assessing their overall physical activity levels during life. Consequently, caution is required when interpreting knee OA as evidence of a highly physically active lifestyle, as well as the absence of knee OA as evidence of a more sedentary lifestyle. In all likelihood, many of our ancient ancestors who developed knee OA did indeed engage in high levels of physical activity, but presumably so did many people who never developed the disease.
Acknowledgments
For help conducting the experiment, we are especially grateful to Pedro Ramirez at Harvard University’s Concord Field Station. For other contributions to the study, we thank Cary Allen-Blevins, Andrew Biewener, Heather Dingwall, Timothy Kistner, Lisa Litchfield, Kathleen Pritchett-Corning, Emmanuel Virot, Kenneth Wilcox, and Dylan Wile. Thanks also to Clinton Rubin for helpful discussions. Funding for this research was provided by the Harvard College Research Program and Hintze Family Charitable Foundation.
References
Austin, A. E. 2017. The cost of a commute: a multidisciplinary approach to osteoarthritis in New Kingdom Egypt. International Journal of Osteoarchaeology 27: 537–550.
Bendele, A. M. 2001. Animal models of osteoarthritis. Journal of Musculoskeletal and Neuronal Interactions 1: 363–376.
Bendele, A. M., R. A. Bendele, J. F. Hulman, and B. P. Swann. 1996. Effets bénéfiques d’un traitement par la diacerhéine chez des cobayes atteints d’arthrose. La Revue du Praticien 46: S35–S39.
Bendele, A. M., and J. F. Hulman. 1988. Spontaneous cartilage degeneration in guinea pigs. Arthritis & Rheumatology 31: 561–565.
Bendele, A. M., and J. F. Hulman. 1991. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis & Rheumatology 34: 1180–1184.
Bendele, A. M., S. L. White, and J. F. Hulman. 1989. Osteoarthritis in guinea pigs: histopathologic and scanning electron microscopic features. Laboratory Animal Science 39: 115–121.
Berenbaum, F., I. J. Wallace, D. E. Lieberman, and D. T. Felson. 2018. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nature Reviews Rheumatology 14: 674–681.
Bramble, D. M., and D. E. Lieberman. 2004. Endurance running and the evolution of Homo. Nature 432: 345–352.
Brandt, K. D., P. Dieppe, and E. Radin. 2009. Etiopathogenesis of osteoarthritis. Medical Clinics of North America 93: 1–24.
Bridges, P. S. 1991. Degenerative joint disease in hunter-gatherers and agriculturalists from the southeastern United States. American Journal of Physical Anthropology 85: 379–391.
Brismar, B. H., W. Lei, A. Hjerpe, and O. Svensson. 2003. The effect of body mass and physical activity on the development of guinea pig osteoarthritis. Acta Orthopaedica Scandinavica 74: 442–448.
Carrier, D. R. 1984. The energetic paradox of human running and hominid evolution. Current Anthropology 25: 483–495.
Cheverko, C. M., and E. J. Bartelink. 2017. Resource intensification and osteoarthritis patterns: changes in activity in the prehistoric Sacramento-San Joaquin Delta region. American Journal of Physical Anthropology 164: 331–342.
Chevillard, L., R. Portet, and M. Cadot. 1963. Growth rate of rats born and reared at 5 and 30 C. Federation Proceedings 22: 699–703.
Driban, J. B., J. M. Hootman, M. R. Sitler, K. P. Harris, and N. M. Cattano. 2017. Is participation in certain sports associated with knee osteoarthritis? A systematic review. Journal of Athletic Training 52: 497–506.
Ewers, B. J., V. M. Jayaraman, R. F. Banglmaier, and R. C. Haut. 2002. Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. Journal of Biomechanics 35: 747–755.
Felson, D. T., J. J. Anderson, A. Naimark, A. M. Walker, and R. F. Meenan. 1988. Obesity and knee osteoarthritis. The Framingham Study. Annals of Internal Medicine 109: 18–24.
Fernandes, L., K. B. Hagen, J. W. J. Bijlsma, O. Andreassen, P. Christensen, P. G. Conaghan, M. Doherty, R. Geenen, A. Hammond, I. Kjeken, L. S. Lohmander, H. Lund, C. D. Mallen, T. Nava, S. Oliver, K. Pavelka, I. Pitsillidou, J. A. da Silva, J. de la Torre, G. Zanoli, and T. P. M. Vliet Vlieland. 2013. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Annals of the Rheumatic Diseases 72: 1125–1135.
Gordon, C. J. 1986. Relationship between behavioral and autonomic thermoregulation in the guinea pig. Physiology & Behavior 38: 827–831.
Griffin, T. M., J. L. Huebner, V. B. Kraus, Z. Yan, and F. Guilak. 2012. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis & Rheumatology 64: 443–453.
Hedrick, T. L. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspiration & Biomimetics 3: 034001.
Helminen, H. J., M. M. Hyttinen, M. J. Lammi, J. P. Arokoski, T. Lapveteläinen, J. Jurvelin, I. Kiviranta, and M. I. Tammi. 2000. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. Journal of Bone and Mineral Metabolism 18: 245–257.
Holowka, N. B., I. J. Wallace, A. Mathiessen, R. M. Ojiambo, P. Okutoyi, S. Worthington, and D. E. Lieberman. 2021. Urbanization and knee cartilage growth among children and adolescents in western Kenya. ACR Open Rheumatology 3: 765–770.
Holowka, N. B., B. Wynands, T. J. Drechsel, A. K. Yegian, V. A. Tobolsky, P. Okutoyi, R. M. Ojiambo, D. W. Haile, T. K. Sigei, C. Zippenfennig, T. L. Milani, and D. E. Lieberman. 2019. Foot callus thickness does not trade off protection for tactile sensitivity during walking. Nature 571: 261–264.
Huebner, J. L., M. A. Hanes, B. Beekman, J. M. TeKoppele, and V. B. Kraus. 2002. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis and Cartilage 10: 758–767.
Hyttinen, M. M., J. P. Arokoski, J. J. Parkkinen, M. J. Lammi, T. Lapveteläinen, K. Mauranen, K. Király, M. I. Tammi, and H. J. Helminen. 2001. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthritis and Cartilage 9: 694–701.
Jurmain, R. D. 1977. Stress and the etiology of osteoarthritis. American Journal of Physical Anthropology 46: 353–366.
Jurmain, R. D. 1999. Stories from the Skeleton: Behavioral Reconstruction in Human Osteology. Amsterdam: Gordon and Breach.
Jurvelin, J., I. Kiviranta, M. Tammi, and H. J. Helminen. 1986. Effect of physical exercise on indentation stiffness of articular cartilage in the canine knee. International Journal of Sports Medicine 7: 106–110.
Kiviranta, I., M. Tammi, J. Jurvelin, A.-M. Säämänen, and H. J. Helminen. 1988. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young Beagle dogs. Journal of Orthopaedic Research 6: 188–195.
Klaus, H. D., C. S. Larsen, and M. E. Tam. 2009. Economic intensification and degenerative joint disease: life and labor on the postcontact north coast of Peru. American Journal of Physical Anthropology 139: 204–221.
Kraft, T. S., V. V. Venkataraman, I. J. Wallace, A. N. Crittenden, N. B. Holowka, J. Stieglitz, J. A. Harris, D. A. Raichlen, B. M. Wood, M. Gurven, and H. Pontzer. 2021. The energetics of uniquely human subsistence strategies. Science 374: eabf0130.
Kraus, V. B., J. L. Huebner, J. DeGroot, and A. Bendele. 2010. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis and Cartilage 18: S35–S52.
Lafortune, M. A., and E. M. Hennig. 1992. Cushioning properties of footwear during walking: accelerometer and force platform measurements. Clinical Biomechanics 7: 181–184.
Larsen, C. S. 1982. The Anthropology of St. Catherine’s Island: 3. Prehistoric Human Biological Adaptation. Anthropological Papers of the American Museum of Natural History, no. 57. New York: American Museum of Natural History.
Larsen, C. S. 2015. Bioarchaeology: Interpreting Behavior from the Human Skeleton, 2nd Edition. Cambridge: Cambridge University Press.
Larsen, C. S., C. B. Ruff, and R. L. Kelly. 1995. Structural analysis of the Stillwater postcranial human remains: behavioral implications of articular joint pathology and long bone diaphyseal morphology. In Bioarchaeology of the Stillwater Marsh: Prehistoric Human Adaptation in the Western Great Basin. Anthropological Papers of the American Museum of Natural History, no. 77, ed. by C. S. Larsen and R. L. Kelly, pp. 107–133. New York: American Museum of Natural History.
Lieverse, A. R., B. Mack, V. I. Bazaliiskii, and A. W. Weber. 2016. Revisiting osteoarthritis in the Cis-Baikal: understanding behavioral variability and adaptation among middle Holocene foragers. Quaternary International 405: 160–171.
Lieverse, A. R., A. W. Weber, V. I. Bazaliiskiy, O. I. Goriunova, and N. A. Savel’ev. 2007. Osteoarthritis in Siberia’s Cis-Baikal: skeletal indicators of hunter-gatherer adaptation and cultural change. American Journal of Physical Anthropology 132: 1–16.
Lo, G. H., J. B. Driban, A. M. Kriska, T. E. McAlindon, R. B. Souza, N. J. Petersen, K. L. Storti, C. B. Eaton, M. C. Hochberg, R. D. Jackson, C. K. Kwoh, M. C. Nevitt, and M. E. Suarez-Almazor. 2017. Is there an association between a history of running and symptomatic knee osteoarthritis? A cross-sectional study from the Osteoarthritis Initiative. Arthritis Care & Research 69: 183–191.
Marlowe, F. W. 2005. Hunter-gatherers and human evolution. Evolutionary Anthropology 14: 54–67.
McAlindon, T. E., R. R. Bannuru, M. C. Sullivan, N. K. Arden, F. Berenbaum, S. M. Bierma-Zeinstra, G. A. Hawker, Y. Henrotin, D. J. Hunter, H. Kawaguchi, K. Kwoh, S. Lohmander, F. Rannou, E. M. Roos, and M. Underwood. 2014. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and Cartilage 22: 363–388.
Milgrom, C., D. Burr, D. Fyhrie, S. Hoshaw, A. Finestone, M. Nyska, R. Davidson, S. Mendelson, M. Giladi, M. Liebergall, B. Lehnert, A. Voloshin, and A. Simkin. 1998. A comparison of the effect of shoes on human tibial axial strains recorded during dynamic loading. Foot & Ankle International 19: 85–90.
Milgrom, C., A. Finestone, S. Segev, C. Olin, T. Arndt, and I. Ekenman. 2003. Are overground or treadmill runners more likely to sustain tibial stress fracture? British Journal of Sports Medicine 37: 160–163.
Newton, P. M., V. C. Mow, T. R. Gardner, J. A. Buckwalter, and J. P. Albright. 1997. The effect of lifelong exercise on canine articular cartilage. American Journal of Sports Medicine 25: 282–287.
Otterness, I. G., J. D. Eskra, M. L. Bliven, A. K. Shay, J. P. Pelletier, and A. J. Milici. 1998. Exercise protects against articular cartilage degeneration in the hamster. Arthritis and Rheumatism 41: 2068–2076.
Paterson, K. L., T. V. Wrigley, K. L. Bennell, and R. S. Hinman. 2014. A survey of footwear advice, beliefs and wear habits in people with knee osteoarthritis. Journal of Foot and Ankle Research 7: 43.
Radin, E. L. 2004. Who gets osteoarthritis and why? The Journal of Rheumatology 70: 10–15.
Radin, E. L., R. D. Boyd, R. B. Martin, D. B. Burr, B. Caterson, and C. Goodwin. 1985. Mechanical factors influencing cartilage damage. In L’Arthrose: Problèmes Cliniques et Fondamentaux Actuels, ed. by J. G. Peyron, pp. 90–99. Paris: Geigy.
Radin, E. L., D. B. Burr, B. Caterson, D. Fyhrie, T. D. Brown, and R. D. Boyd. 1991. Mechanical determinants of osteoarthrosis. Seminars in Arthritis and Rheumatism 21: 12–21.
Radin, E. L., R. B. Orr, J. L. Kelman, I. L. Paul, and R. M. Rose. 1982. Effect of prolonged walking on concrete on the knees of sheep. Journal of Biomechanics 15: 487–492.
Radin, E. L., I. L. Paul, and R. M. Rose. 1972. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet 299: 519–522.
Robbins, A., C. Tom, M. N. Cosman, C. Moursi, L. Shipp, T. M. Spencer, T. Brash, and M. J. Devlin. 2018. Low temperature decreases bone mass in mice: implications for humans. American Journal of Physical Anthropology 167: 557–568.
Rothschild, B. M. 2003. Osteoarthritis as a complication of artificial environment: the Cavia (guinea pig) story. Annals of the Rheumatic Diseases 62: 1022–1023.
Säämänen, A.-M., M. Tammi, I. Kiviranta, and H. J. Helminen. 1988. Running exercise as a modulatory of proteoglycan matrix in the articular cartilage of young rabbits. International Journal of Sports Medicine 9: 127–33.
Säämänen, A.-M., M. Tammi, I. Kiviranta, J. Jurvelin, and H. J. Helminen. 1989. Levels of chondroitin-6-sulfate and nonaggregating proteoglycans at articular cartilage contact sites in the knees of young dogs subjected to moderate running exercise. Arthritis & Rheumatology 32: 1282–1292.
Serrat, M. A. 2014. Environmental temperature impact on bone and cartilage growth. Comprehensive Physiology 4: 621–655.
Timmins, K. A., R. D. Leech, M. E. Batt, and K. L. Edwards. 2017. Running and knee osteoarthritis: a systematic review and meta-analysis. American Journal of Sports Medicine 45: 1447–1457.
Turner, A. P., J. H. Barlow, M. Buszewicz, A. Atkinson, and G. Rait. 2007. Beliefs about the causes of osteoarthritis among primary care patients. Arthritis and Rheumatism 57: 267–271.
Vaanholt, L. M., T. Garland Jr., S. Daan, and G. H. Visser. 2007. Wheel-running activity and energy metabolism in relation to ambient temperature in mice selected for high wheelrunning activity. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 177: 109–118.
Wallace, I. J., A. M. Bendele, G. Riew, H.-H. Hung, E. H. Frank, N. B. Holowka, A. S. Bolze, E. M. Venable, A. K. Yegian, H. L. Dingwall, R. N. Carmody, A. J. Grodzinsky, and E. E. Lieberman. 2019. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthritis and Cartilage 27: 1721–1728.
Wallace, I. J., E. Koch, N. B. Holowka, and D. E. Lieberman. 2018. Heel impact forces during barefoot versus minimally shod walking among Tarahumara subsistence farmers and urban Americans. Royal Society Open Science 5: 180044.
Wallace, I. J., G. J. Riew, R. Landau, A. M. Bendele, N. B. Holowka, T. L. Hedrick, N. Konow, D. J. Brooks, and D. E. Lieberman. 2022. Experimental evidence that physical activity inhibits osteoarthritis: implications for inferring activity patterns from osteoarthritis in archeological human skeletons. American Journal of Biological Anthropology 177: 223–231.
Wallace, I. J., S. Worthington, D. T. Felson, R. D. Jurmain, K. T. Wren, H. Maijanen, R. J. Woods, and D. E. Lieberman. 2017. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proceedings of the National Academy of Sciences USA 114: 9332–9336.
Whittle, M. W. 1999. Generation and attenuation of transient impulsive forces beneath the foot: a review. Gait & Posture 10: 264–275.
Wluka, A. E., C. B. Lombard, and F. M. Cicuttini. 2013. Tackling obesity in knee osteoarthritis. Nature Reviews Rheumatology 9: 225–235.
Yang, K. H., R. D. Boyd, V. L. Kish, D. B. Burr, B. Caterson, and E. L. Radin. 1989. Differential effect of load magnitude and rate on the initiation and progression of osteoarthritis. Transactions of the Orthopaedic Research Society 14: 148.
Zapata-Linares, N., F. Eymard, F. Berenbaum, and X. Houard. 2021. Role of adipose tissues in osteoarthritis. Current Opinion in Rheumatology 33: 84–93.
Zipser, B., A. Schleking, S. Kaiser, and N Sachser. 2014. Effects of domestication on biobehavioural profiles: a comparison of domestic guinea pigs and wild cavies from early to late adolescence. Frontiers in Zoology 11: 30.